Figure 3. The primary and secondary rod pathways, not cone light responses, drive the photopic PLR.
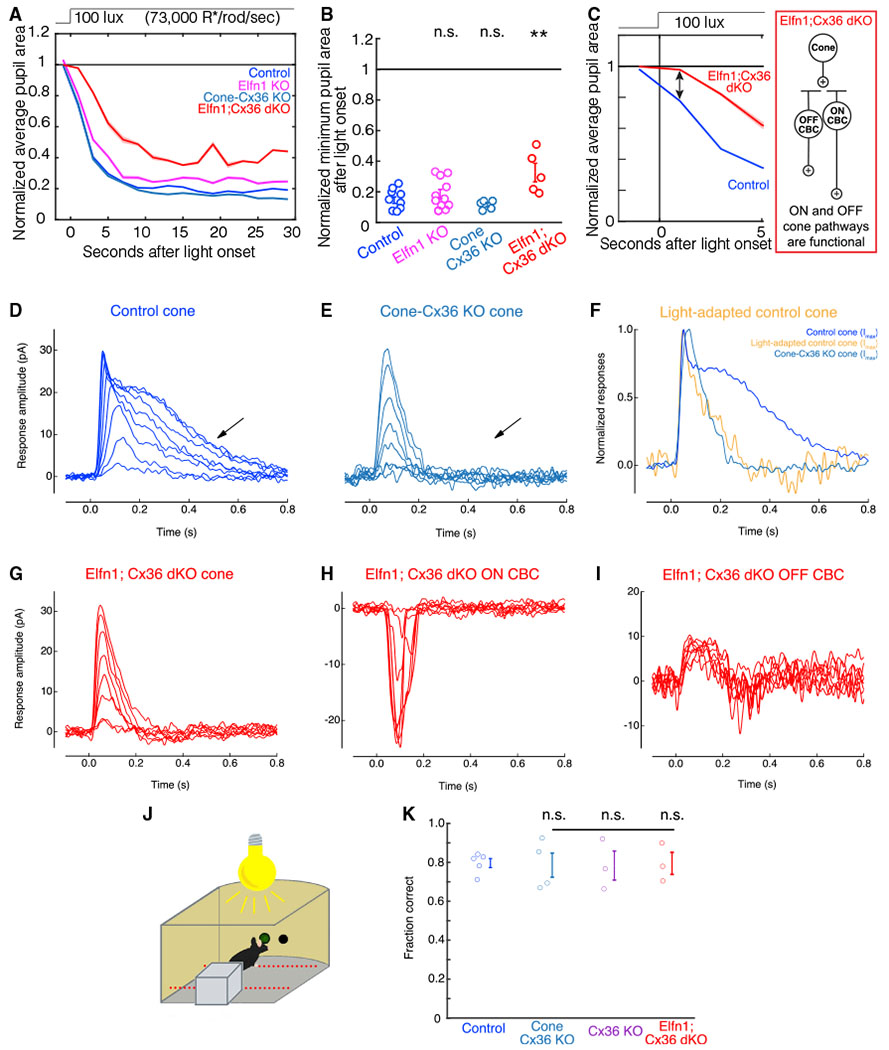
(A) The average pupil constriction over time in response to 100-lux light (73,000 R*/rod/s) beginning at t = 0 s (dashed line) for control (blue, n = 9), Elfn1 KO (magenta, n = 11), Cone-Cx36 KO (cyan, n = 5), and Elfn1; Cx36 dKO (red, n = 5) mice. Shaded outlines represent SEM. All pupil sizes are normalized to the dark-adapted pupil size (before t = 0).
(B) The minimum pupil area (maximal constriction) in response to 100-lux light from t = 0 to t = 30 s. Individuals and SEM are shown. Significance from the control group is as follows: Elfn1 KO, p = 0.58; Cone-Cx36 KO, p = 0.89; Elfn1; Cx36 dKO, p = 0.003 (ANOVA post hoc Dunnett’s method).
(C) The PLR delay seen in (A). Elfn1; Cx36 dKO mice (red) have a pupil constriction deficit immediately after light onset compared with control mice (ANOVA post hoc Dunnett’s method, p = 0.04). Cone pathways are functional in these mice, as indicated by the circuit diagram.
(D–F) Physiological recordings of cone photocurrents in retinal slices from littermate control and Cone-Cx36 KO mice. Recordings were made in whole-cell patch-clamp mode (Vm = −40 mV). 20-ms light flashes were given at time 0 s. Flash strength ranged from 3.1–203.4 R*/rod.
(D) In recordings from cones in control mice, a fast transient peak is followed by a slower sustained rod-dependent (arrow) response.
(E) In recordings from cones in Cone-Cx36 KO mice, the fast transient peak remains, but the slower sustained response is missing (arrow).
(F) A rod-suppressing background light (332 R*/rod/s) was applied to retinal slices in littermate control mice. In recordings from cones after light adaptation, the fast transient peak remains, but the slower sustained response is missing (yellow). Normalized responses from dark-adapted cones in Cone-Cx36 KO (cyan) and littermate control (blue) mice are shown for comparison.
(G–I) Physiological recordings of photopic light responses recorded in an Elfn1; Cx36 dKO retina slice. Recordings were the same as in (D), except that flash strength ranged from 31–2,034 R*/rod. Recordings are representative of data collected across several cells.
(G) Cone responses in Elfn1; Cx36 dKO mice are missing the rod-dependent sustained response.
(H) ON CBCs depolarize in response to a photopic light flash.
(I) OFF CBCs hyperpolarize in response to a photopic light flash.
(J) Schematic depicting the visually guided behavioral task with a rod-suppressing background light (3,500 R*/rod/s) illuminating the testing chamber. The light stimulus was presented at photopic levels (28,000 R*/rod/s).
(K) Quantification of the testing trials by control mice (blue, n = 5), Cone-Cx36 KO mice (cyan, n = 4), Cx36 KO mice (purple, n = 3), and Elfn1; Cx36 dKO mice (red, n = 3) under rod-suppressing background light. KO mice correctly detect the light stimulus with an accuracy comparable with controls under light-adapted conditions (ANOVA post hoc Dunnett’s method, KO mice from control mice, all p > 0.99). Error bars indicate SEM.
