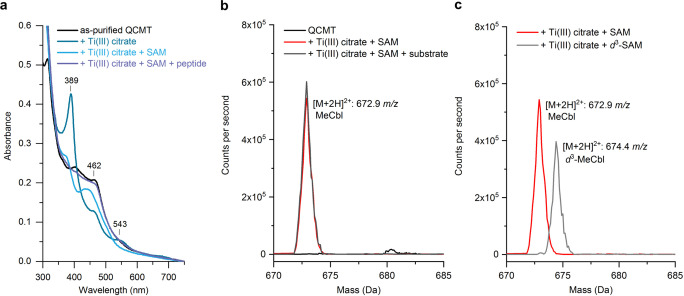
Figure 6.
Characterization of QCMT‐bound cobalamin. a) UV/Vis absorption spectra of 20 μM as‐purified QCMT, 20 μM as‐purified QCMT treated with 80 μM titanium(III) citrate, 20 μM as‐purified QCMT treated with 80 μM titanium(III) citrate and 40 μM SAM, 20 μM as‐purified QCMT treated with 80 μM titanium(III) citrate, 40 μM SAM and 20 μM peptide substrate. Characteristic absorption bands of the titanium(III) citrate reduced QCMT are indicated. b) QCMT contains MeCbl in the presence of SAM under reducing conditions. LC‐MS/MS spectrum showing all precursor ions that fragment to the mass of unmethylated cobalamin (665.3 m/z, [M+2 H]2+). QCMT‐bound MeCbl was detected after addition of titanium(III) citrate and excess SAM from an enzymatic regeneration system. c) Isotopic labeling shows that QCMT‐bound cobalamin is methylated from externally supplied SAM. LC‐MS/MS spectrum showing all precursor ions that fragment to the mass of unmethylated cobalamin (665.3 m/z, [M+2 H]2+). In the presence of externally supplied d 3‐SAM produced from d 3‐methionine, QCMT‐bound d 3‐MeCbl was detected, as evidenced by a mass shift of +1.5 Da for the [M+2 H]2+ peak.