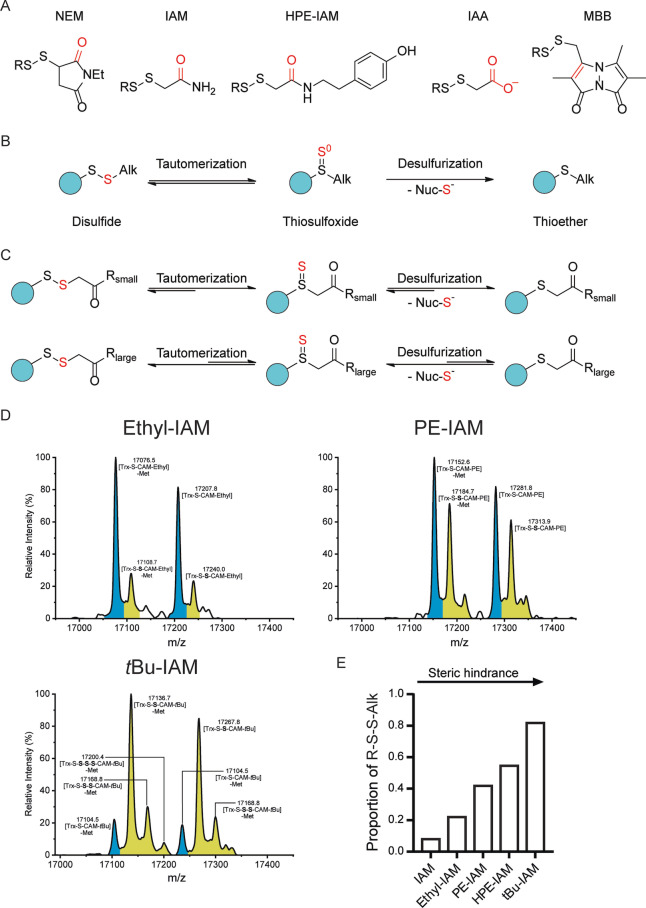
Figure 4.
The concept of disulfide–thiosulfoxide tautomerism predicts differences in the stability of alkylated persulfides. A) Structures of alkylated persulfides (R‐S‐S‐Alk) as formed with the alkylating agents NEM, IAM, IAM‐HPE, IAA and MBB (from left to right). B) Proposed mechanism of sulfur loss from alkylated persulfides: Disulfide‐thiosulfoxide tautomerism of alkylated persulfides is coupled to nucleophile‐mediated desulfurization of the thiosulfoxide, generating the thioether. C) Proposed influence of the bulkiness of the substituent in γ‐position on the efficiency of tautomerization and/or desulfurization. D) Whole protein mass spectra of Trx1CS (13.5 μM) persulfidated with Na2S2 (300 μM) and alkylated with Ethyl‐IAM (upper left panel), PE‐IAM (upper right panel) or N‐t‐butyl‐IAM (lower left panel) (1 mM each). Peaks corresponding to unmodified or thiol‐alkylated proteins are highlighted in blue and peaks corresponding to per‐/polysulfide‐alkylated proteins are highlighted in yellow. E) Relative proportion of recovered R‐S‐S‐Alk species for different IAM derivatives arranged in order of increasing γ‐substituent bulkiness.