Figure 5.
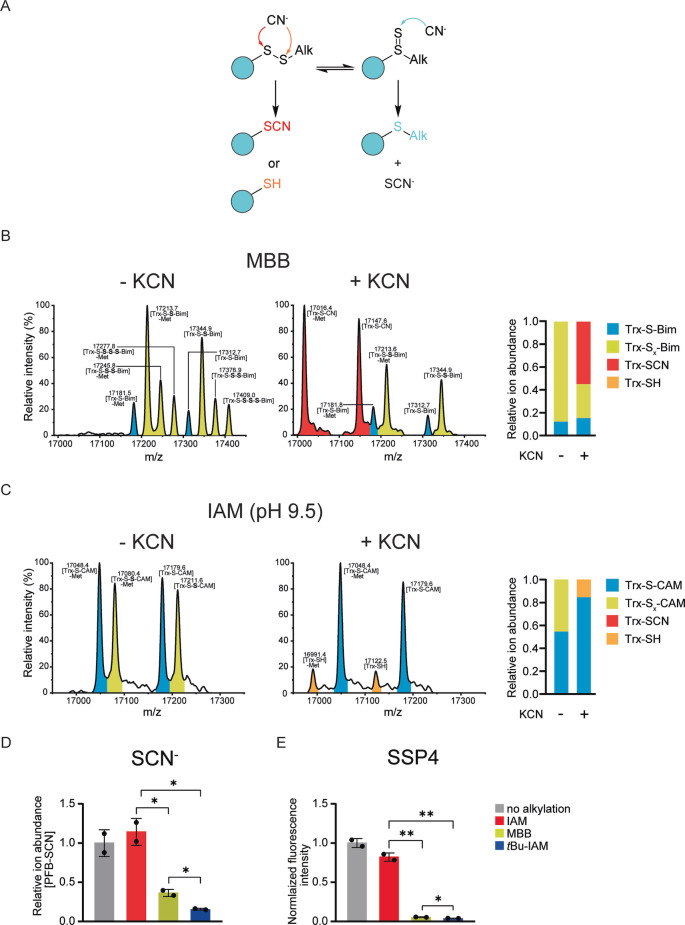
Labile alkylated persulfides lose sulfur to nucleophilic acceptors. A) Expected reactions of cyanide with the two different tautomeric forms of alkylated persulfides. B) Whole protein mass spectra of Trx1CS (13.5 μM) persulfidated with Na2S2 (300 μM), alkylated with MBB (1 mM), and then treated with buffer (left panel) or KCN (0.5 mM) (middle panel). Quantification of relative species abundance (right panel). C) Whole protein mass spectra of Trx1CS persulfidated with Na2S2, alkylated with IAM (1 mM) at pH 9.5, and then treated with buffer (left panel) or KCN (0.5 mM) (middle panel). Quantification of relative species abundance (right panel). B), C) Peaks corresponding to thiol‐alkylated proteins are highlighted in blue and peaks corresponding to per‐/polysulfide‐alkylated proteins are highlighted in yellow. In addition, peaks corresponding to Trx1CS‐SCN are highlighted in red and peaks corresponding to Trx1CS‐SH are highlighted in orange. D) GC‐MS based quantification of thiocyanate (SCN−) released from alkylated persulfides by treatment with cyanide, n=2. E) Quantification of sulfane sulfur released from alkylated persulfides by treatment with the fluorogenic sulfane sulfur acceptor SSP4 (100 μM), n=3. Error bars represent SD, * P≤0.05; ** P≤0.01, based on a two‐tailed unpaired t‐test.