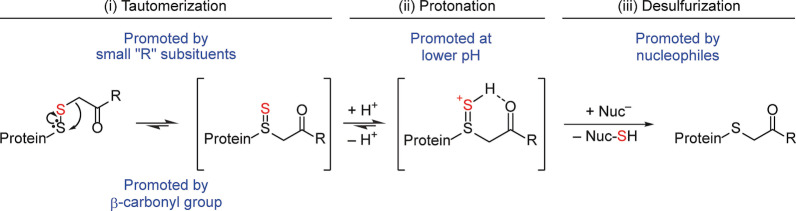
Figure 8.
Possible mechanism of alkylating agent‐driven sulfur loss. (i) The thiosulfoxide may form by migration of the S‐C bond to the ‘internal’ sulfur with concomitant expansion of that sulfur's coordination number to 4. (ii) Shared sulfane sulfur–carbonyl oxygen proton binding may support the formation and stabilization of the thiosulfoxide. (iii) The cationic intermediate is expected to lose sulfur to nucleophiles more favorably than the corresponding non‐protonated species. The figure also points out the four factors promoting sulfur loss as identified in this study, i.e., two factors intrinsic to the alkylating agent (a carbonyl in β‐position and small substituents in γ‐position) and two extrinsic factors (proton and nucleophile availability).