Figure 3.
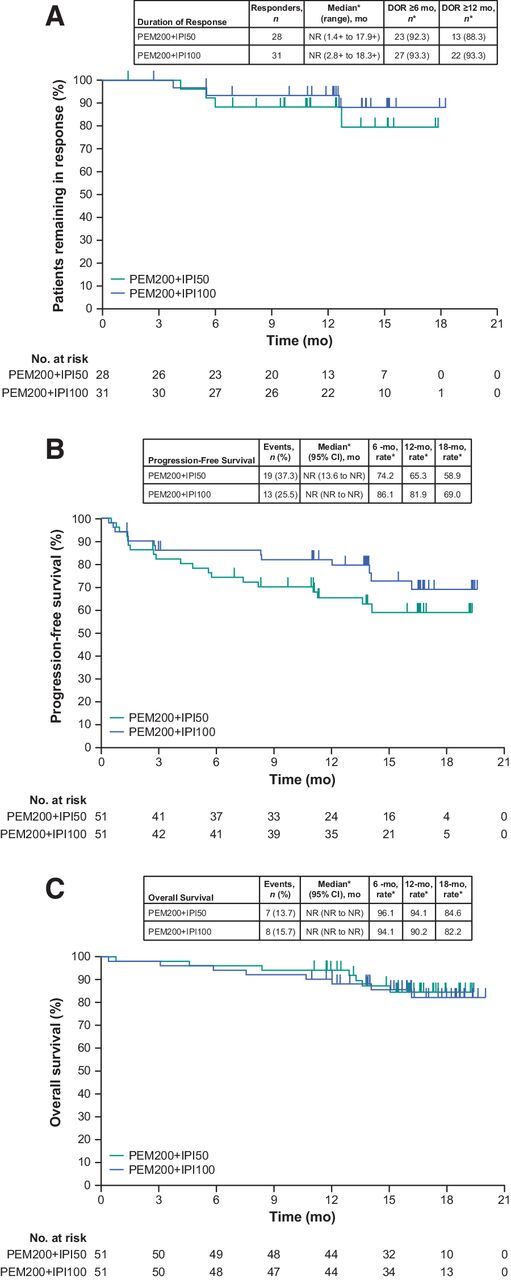
Kaplan–Meier estimates of (A) duration of response, (B) progression-free survival, and (C) overall survival in PEM200+IPI50 (pembrolizumab 200 mg Q3W + ipilimumab 50 mg Q6W) and PEM200+IPI100 (pembrolizumab 200 mg Q3W + ipilimumab 100 mg Q12W) arms. *From Kaplan–Meier method. Abbreviations: DOR, duration of response; NR, not reached; Q3W, every 3 weeks; Q6W, every 6 weeks; Q12W, every 12 weeks.
