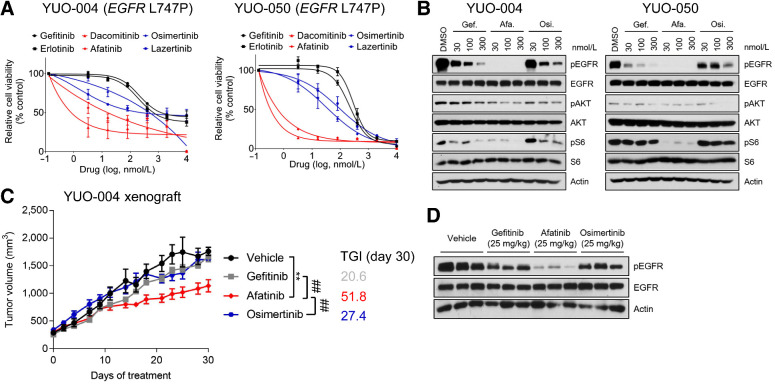
Figure 5.
PDOs predict clinical activity of afatinib against EGFR L747P mutation. A, YUO-004 and YUO-050 were treated with the indicated concentrations of gefitinib, erlotinib, dacomitinib, afatinib, osimertinib, and lazertinib for 3 days. First-generation EGFR-TKIs are colored in dark, second-generation EGFR-TKIs are in red, and third-generation EGFR-TKIs are in blue. Data are presented as the mean ± SEM (n = 3). B, Representative immunoblots of indicated molecules in YUO-004 and YUO-050 treated with the indicated concentrations of gefitinib, afatinib, and osimertinib for 6 hours. C, Tumor growth curve of YUO-004 xenografts treated with indicated drugs at 25 mg/kg once daily (n = 6 per group; one-way ANOVA with Dunnett's posttest: n.s., not significant; **, P < 0.005 vs. vehicle; ##, P < 0.01 vs. afatinib). D, Immunoblots of indicated molecules in tumor samples obtained from YUO-004 xenografts treated with vehicle and 25 mg/kg gefitinib, afatinib, and osimertinib for 30 days.