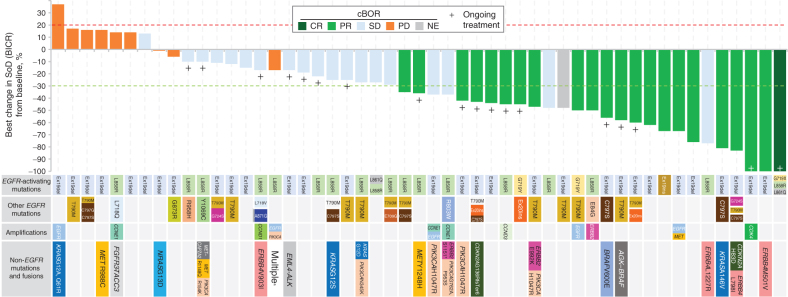
Figure 1.
Best percentage change in the tumor sum of diameters (SoD) from baseline for the pooled HER3-DXd (5.6 mg/kg, i.v. once every 3 weeks) population. Tumor genomic alterations prior to treatment with HER3-DXd are provided for each patient. Six patients could not be evaluated for BOR due to lack of adequate post–baseline tumor assessment and are not shown; one patient had a BOR of NE due to achieving SD too early (<5 weeks) and is shown with hatched markings. Genomic analysis was performed centrally using Oncomine Comprehensive Assay v3 (Thermo Fisher Scientific). Results from local testing are included, if available, together with any additional mutations detected using GuardantOMNI assay of ctDNA in blood collected prior to treatment with HER3-DXd. For ctDNA analysis, a minor allelic frequency of ≥0.1% was used as a threshold for detection of mutations. aPatient had multiple tumor mutations comprising CDKN2A A143V; PIK3CA E542K, E545K, E726K; ERBB2 K200N; and ERBB3 Q847*, Q849*. cBOR, Confirmed BOR.