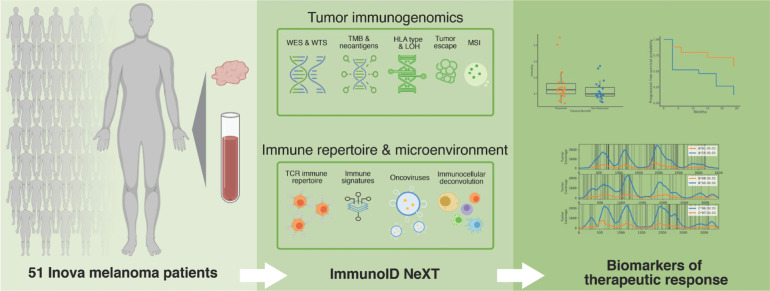
Figure 1.
Study schema. Pretreatment blood normal and tumor samples were collected from 51 patients with unresectable, stage III/IV melanoma who underwent anti–PD-1 therapy. Samples were profiled using Personalis' ImmunoID NeXT platform, an enhanced exome/transcriptome platform and analysis pipeline, which produces comprehensive tumor mutation information, gene expression quantification, neoantigen characterization, HLA (typing, mutation, and LOH), TCR repertoire profiling, MSI detection, oncovirus identification, and TME profiling. These data were then analyzed together with clinical outcome, and a composite neoantigen score computed for each patient along with additional biomarkers, such as TMB.