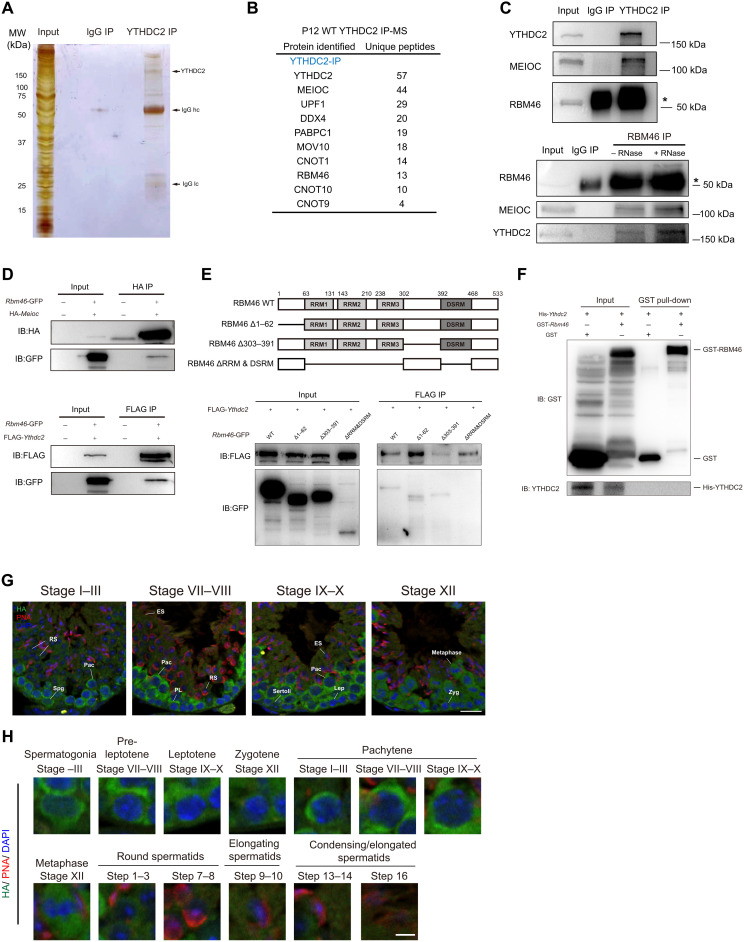
Fig. 1. RBM46 associates with YTHDC2 and MEIOC in mouse testis.
(A) Identification of YTHDC2-associated partners by immunoprecipitation (IP) experiments with either YTHDC2 or normal immunoglobulin antibodies from P12 mouse testes. Immunoprecipitated YTHDC2 protein complexes were separated by SDS-PAGE, and the gel was stained with silver before mass spectrometry (MS). Testis lysates were prepared from mouse at P12 (n = 8). (B) Selective YTHDC2-associated protein candidates identified by MS analysis from P12 mouse testes. (C) Immunoprecipitation of YTHDC2 from mouse testis lysates at P21 and Western blot with anti-YTHDC2, anti-MEIOC, and anti-RBM46 antibodies (top). RBM46 protein was immunoprecipitated with anti-RBM46 antibodies with or without RNases, followed by Western blot analysis with indicated antibodies (bottom). (D) Full-length GFP-Rbm46 and FLAG-Ythdc2 or HA-Meioc expression constructs were cotransfected in HEK293T cells. Cell lysates were immunoprecipitated with anti-FLAG or anti-HA antibodies and Western blot analysis with indicated antibodies. IB, immunoblotting. (E) GFP-tagged RBM46 mutants with deleted fragments were coexpressed with FLAG-YTHDC2 in HEK293T cells. Cell lysates were immunoprecipitated with anti-FLAG antibodies before Western blot analysis with GFP antibodies. (F) GST pull-down assay showing that in vitro translated YTHDC2 does not bind to GST-RBM46. GST-RBM46 was expressed in E. coli and purified with glutathione beads. YTHDC2 with N-terminal His tag was in vitro translated. (G and H) Immunofluorescence analysis of Rbm46-HA knock-in mice with anti-HA antibodies and the acrosome marker PNA that defines different stages of spermatogenesis. DNA was counterstained with 4′,6-diamidino-2-phenylindole (DAPI). Scale bars, 20 μm. Lower panels show magnification image of single cell in the upper panels. Spg, spermatogonia; PL, pre-leptotene; Lep, leptotene; Zyg, zygotene; Pac, pachytene; RS, round spermatids; ES, elongating spermatids. Scale bars, 5 μm.