Extended Data Figure 8. Binding to PI4P on dTGN is essential for NLRP3 inflammasome activation.
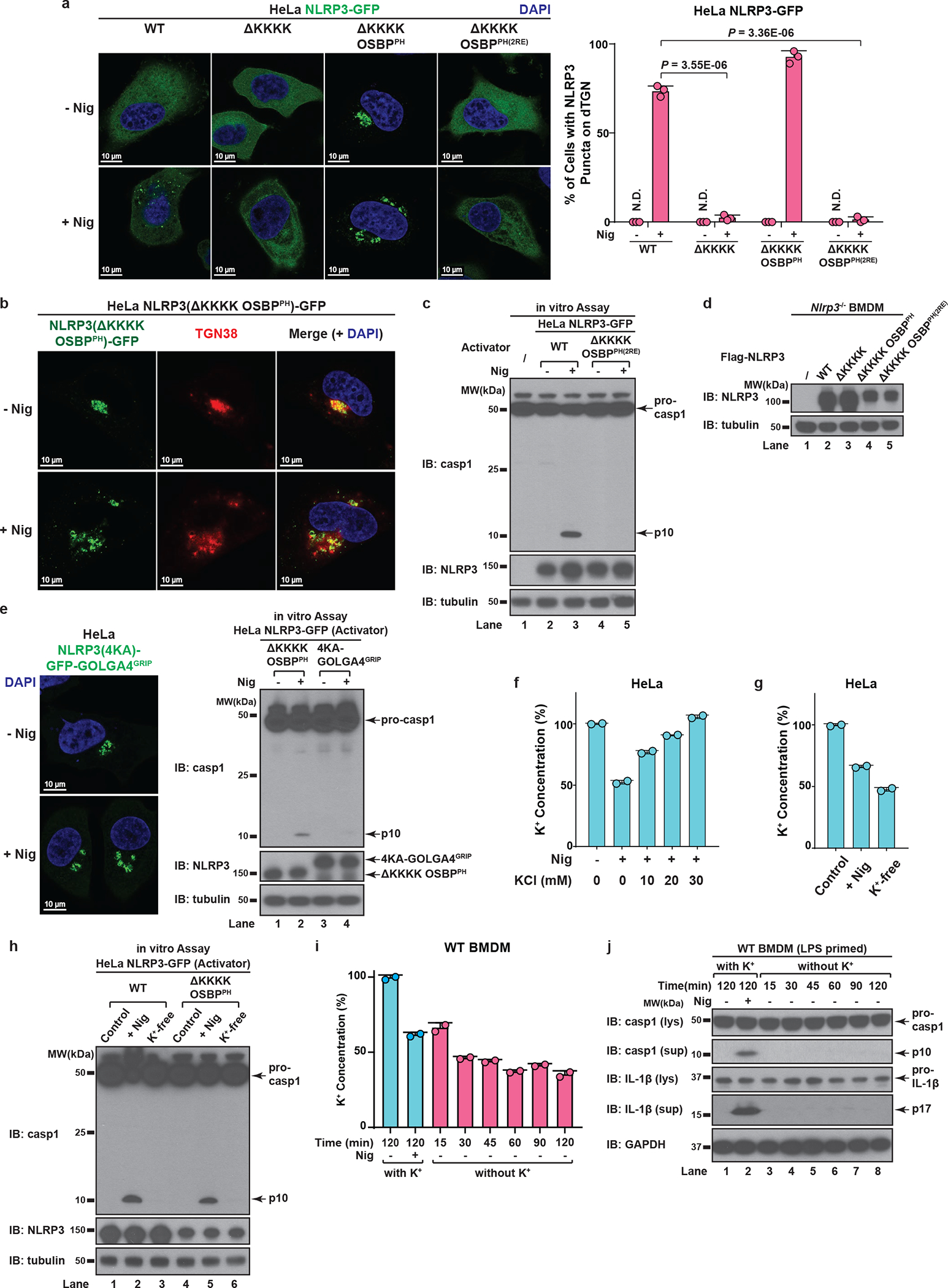
a, b, The KKKK motif of NLRP3 can be functionally replaced with a PI4P-binding domain of OSBP. (a) Cells stably expressing the indicated proteins were treated −/+ nigericin (10 μM) for 80 min before imaging. The percentage of cells with NLRP3 on dTGN was quantified from 100 cells (n = 3, mean ± SD, two-sided t test). N.D., not detectable. 2RE, R107/108E. Note that NLRP3(ΔKKKK OSBPPH) constitutively localized on intact TGN without stimulation was not counted in the quantification. (b) Replacement of the KKKK motif with OSBPPH domain allowed NLRP3 to be constitutively localized on TGN. Cells were treated as in (a) before immunostained for TGN38. c, Mutations of OSBP PH domain that abrogates its PI4P binding also abolish its ability to functionally rescue NLRP3(ΔKKKK). Cells were treated as in (a) before extracts were examined by the in vitro NLRP3 activity assay. d, Immunoblots for primary NLRP3-deficient BMDMs rescued with Flag-NLRP3 (WT or mutants), which were used for experiment in Fig. 5b and 6d. The cells were infected with lentivirus encoding the indicated proteins for 6 days before immunoblotting. e, Recruitment of NLRP3 to non-PI4P-enriched regions of TGN is not sufficient to support its activation. Cells stably expressing the indicated proteins were treated as in (a) before examined by fluorescence microscopy (left panel; images for NLRP3(ΔKKKK OSBPPH) are shown in (a)) and the in vitro assay (right panel). f, Extracellular KCl at 30 mM was sufficient to completely block nigericin-induced K+ efflux. Cells were treated −/+ nigericin (10 μM) for 80 min in the presence of increasing concentrations of KCl, before cell extracts were collected for measurement of intracellular K+ concentration (shown as percentage change compared to untreated sample, mean ± SD). Representative results from two independent experiments (each contains two samples for each condition) are shown. g, Incubation in K+-free medium induced spontaneous K+ efflux. Cells were incubated in Hanks buffer containing 5 mM K+ for the first two conditions, or Hanks buffer without K+ (replaced by Na+) for the third condition (K+-free). The second condition also contained nigericin (10 μM). After 80 min, the cell extracts were collected for intracellular K+ measurement with methods similar to (f). h, K+ efflux alone is not sufficient to activate either WT NLRP3 or NLRP3(ΔKKKK OSBPPH). Cells stably expressing the indicated proteins were treated as in (g) before examined by the in vitro NLRP3 activity assay. i, Incubation in K+-free medium induced spontaneous K+ efflux in primary WT BMDMs. Cells were primed with LPS (50 ng/mL) for 3 hours, before treatment as in (g) with the indicated time lengths. Intracellular K+ concentrations were then measured and analyzed as in (f). j, K+ efflux alone is not sufficient to activate endogenous NLRP3 in primary WT BMDMs. Cells treated as in (i) were used for immunoblotting. lys, lysate; sup, supernatant.
