Extended Data Fig. 10. The lower activity of recombinant Polα-primase cannot be explained entirely by weaker binding to the ssDNA template and reconstitution of telomere end-replication.
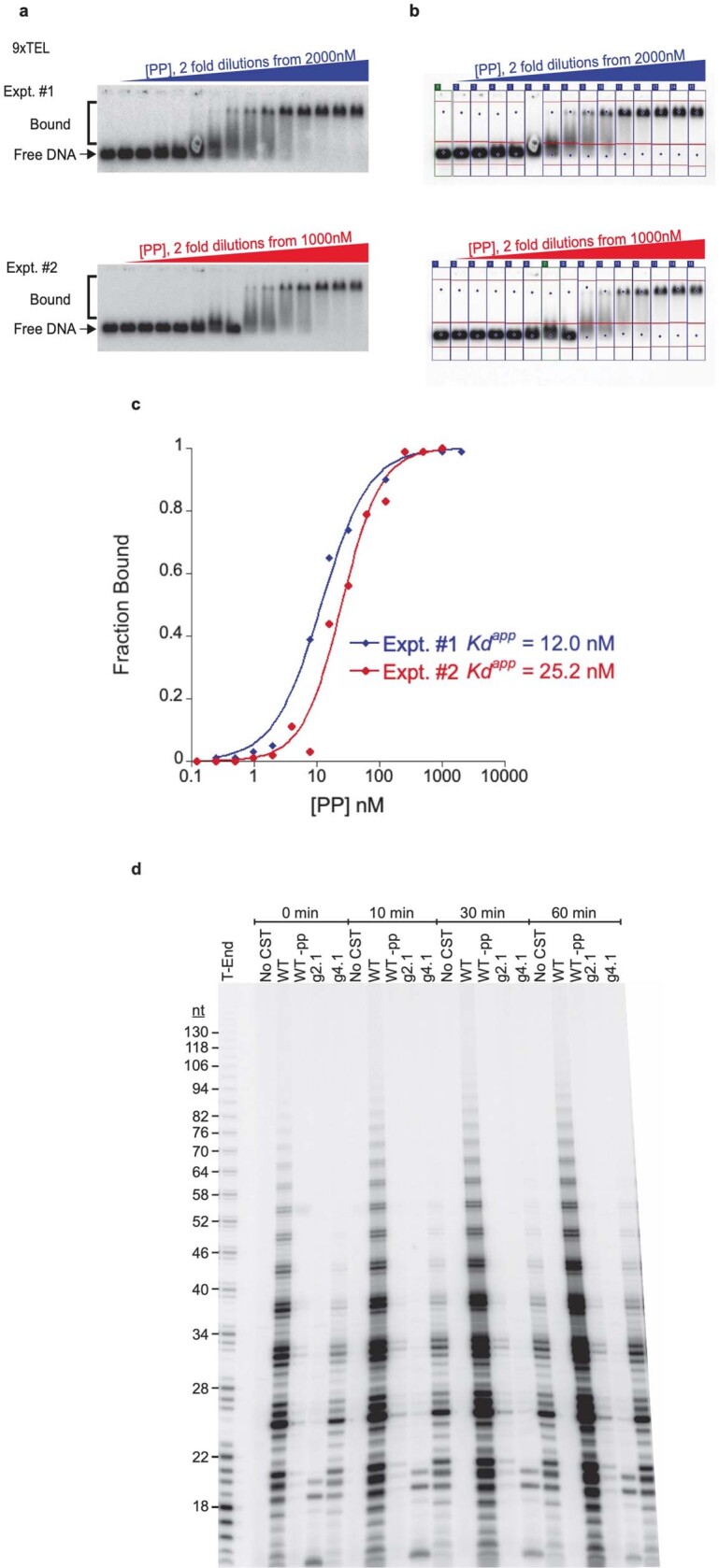
a, Two EMSA experiments measuring binding of Polα-primase purified from insect cells (Insect PP in Extended Data Fig. 1) with labelled 9xTEL DNA. b, Because these DNA-PP complexes dissociate during electrophoresis, the definition we used for free and bound DNA is shown here by the red horizontal lines. The bound species are taken to include both the fully bound complex and the smear of counts between bound and free DNA. c, Quantification of fraction bound as a function of Polα-primase concentration, performed as in Extended Data Fig. 8b. Although the apparent Kd values determined here for Insect cell PP are similar to those determined in Extended Data Fig. 8 for HEK cell CST-PP, recall that CST-PP is only 21% PP. Thus, in terms of PP binding, the CST-PP has 5-fold higher affinity. d, Reconstitution of complete telomere end-replication. C-strands were labelled with α-32P-dCTP. Time between initiation of telomerase reaction and addition of CST–Polα-primase is indicated at top. CST–Polα-primase is present at 75 nM. WT-pp is CST largely depleted of Polα-primase by conducting IP purification at high (300 mM) salt concentration. g2.1 is a DNA-binding defective mutant of CST, and g4.1 is a control mutant of CST that retains DNA binding but is present at only one-third the concentration as WT CST owing to lower yield during purification. For gel source data, see Supplementary Fig. 1
