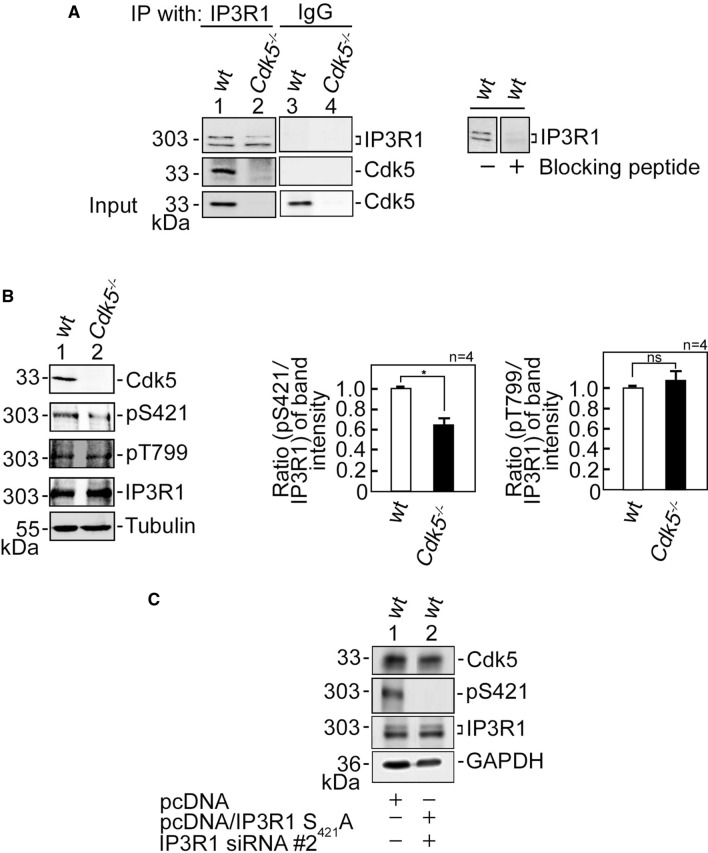
Fig. 3.
Cdk5 associates with and phosphorylates IP3R1 at Ser421. A Cdk5 associates with IP3R1. Lysates of wt and Cdk5−/− MEFs were subjected to immunoprecipitation (IP) using IP3R1 antibody. The IPs were resolved by 4–20% gradient SDS-PAGE and then immunoblotted for IP3R1 and Cdk5 (left panel). To assess the specificity of the IP3R1 antibody, lysates of wt MEFs were blotted (right panel) with antibody blocked (lane 2) or not blocked (lane 1) with the peptide antigen that was used to raise the antibody. Lanes 3 and 4 represent IP control using normal IgG. B Cdk5 specifically phosphorylates IP3R1 at Ser421. Lysates of wt and Cdk5−/− MEFs were subjected to SDS-PAGE and then immunoblotted for IP3R1 phosphoSer421 and phosphoThr799, IP3R1, Cdk5 and tubulin. Tubulin blot was used as loading control. Representative blots are from one of four independent experiments (n = 4) showing similar results. Ratios of levels of IP3R1 phosphoSer421 (middle panel) and phosphoThr799 (right panel) vs total IP3R were calculated following densitometric analysis of blots using NIH Image J 1.61. Standard deviations were calculated based on the ratios obtained from the four independent sets of experiments. Values from wt MEFs were normalized to 1.0. *p < 0.05. ns: not significant. C Shows the specificity of the IP3R1 phosphoSer421 antibody. Lysates of wt MEFs depleted of endogenous IP3R1, but expressing exogenous IP3R1 S421A (res) were subjected to immunoblotting for IP3R1 phosphoSer421, IP3R1, and Cdk5. GAPDH blot was used as loading control