Fig. 4 |. Substrate-bound structures of AAA+ proteins reveal a conserved hand-over-hand substrate translocation mechanism.
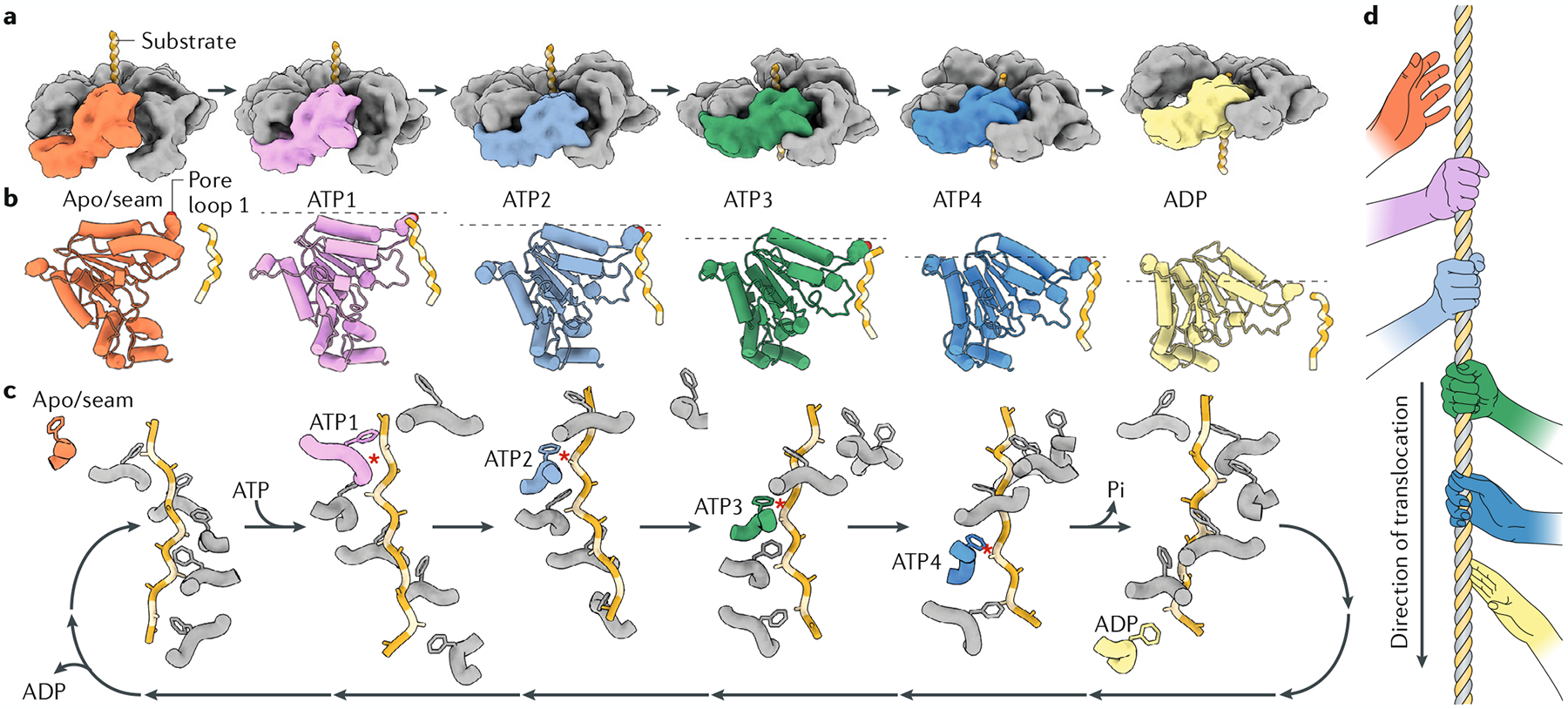
a | Side view of the ATPase associated with diverse cellular activities (AAA+) staircase with a single subunit coloured in each panel to show how it progresses through consecutive positions in the staircase, translocating the substrate with it. Each distinct colour corresponds to the unique position adopted within the hexamer during the translocation cycle. The cycle begins with the subunit that is unbound to nucleotide (apo/seam, coloured orange), and thus displaced from the hexamer and not interacting with the substrate. Upon ATP binding, the subunit progresses through four registers of the spiral staircase within the hexamer (ATP1, pink; ATP2, light blue; ATP3, green; ATP4, dark blue), until hydrolysis in the nucleotide-binding side triggers the subunit to release from the substrate (ADP, coloured yellow). The concerted movement of all six subunits within the hexamer progressing through this cycle sequentially results in hand-over-hand translocation of the substrate. b | The corresponding coloured subunit from part a is shown using a ribbon representation to demonstrate how the ATPase domain progressively tilts downwards through the translocation cycle, giving rise to a downward motion of pore loop 1 (the pore loop 1 aromatic residue is shown using a sphere representation) and the substrate. The dotted grey line emphasizes the downward progression of each step through the cycle. c | Pore loops of the six subunits in an AAA+ hexamer assemble into a spiral staircase that wraps around the translocating substrate. Any given subunit (we follow the cycle of one subunit, coloured according to its register in the ATPase hexamer, as defined in part a) transitions through each position of the staircase hydrolysing ATP at the bottom of the staircase, releasing ADP and detaching from the substrate as it resets to assume a position at the top of the staircase upon rebinding of ATP. Substrate-engaged states are indicated by a red star between the pore loop 1 residue and the substrate. d | Cartoon illustrating the hand-over-hand mechanism of substrate translocation between the subunits of an ATPase associated with diverse cellular activities (AAA+ protein).
