Fig. 5 |. The universal substrate-interacting pore loop 1 is uniquely adjusted in different AAA+ proteins.
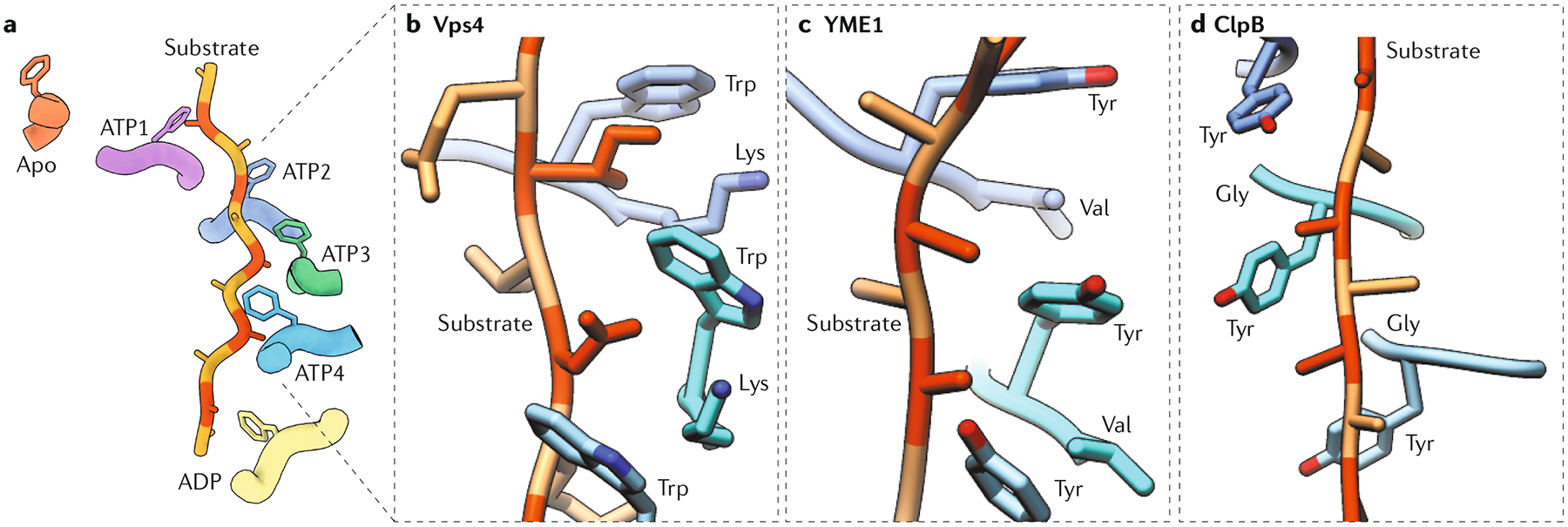
a | The pore loop 1 aromatic residue of the ATP-bound subunits (ATP1–ATP4) intercalates against the substrate every two amino acids, whereas the pore loop of the ADP-bound subunit has limited interactions with the substrate and the apo subunit does not contact the substrate. The pore loop 1–substrate interactions are mediated by the conserved aromatic residue of pore loop 1 of each subunit (see parts b–d where these residues in pore loop 1 of subunits ATP2–ATP4 are shown). b–d | The overall spiralling organization and molecular principles of the pore loop–substrate interface are conserved among classical AAA+ domains (as well as some non-classical domains, part d), but non-conserved residues within pore loop 1 give rise to unique environments around the translocating substrate in different ATPases associated with diverse cellular activities (AAA+ proteins). For example, like in most classical clade AAA+ proteins, the residue preceding this aromatic residue (aromatic-prior position) corresponds to lysine (Lys) in Vps4 (pdb:6BMF, part b). This basic residue engages in cation–π interactions with the conserved aromatic both in cis (within the same subunit) and in trans (with a neighbouring subunit). This configuration likely increases stability of the staircase and strengthens inter-subunit communication. Similar to Vps4, YME1 (pdb:6AZ0, part c) engages the substrate through the pore loop aromatic, which corresponds to tyrosine (Tyr) in this case. However, the aromatic-prior residue corresponds to valine (Val) instead of lysine, which increases the hydrophobicity around the translocating substrate, but at the same time eliminates the stabilizing cation–π interactions within the ATPase staircase. Domain 2 of ClpB (pdb:6OAY, part d) — a domain originating from the HCLR clade — contains glycine (Gly) as an aromatic-prior residue. In consequence, this increases flexibility within the pore loop staircase, which likely weakens the organization of the staircase.
