Fig. 6 |. Distinct structural features of different of AAA+ proteins mediate additional interactions with the translocating substrate.
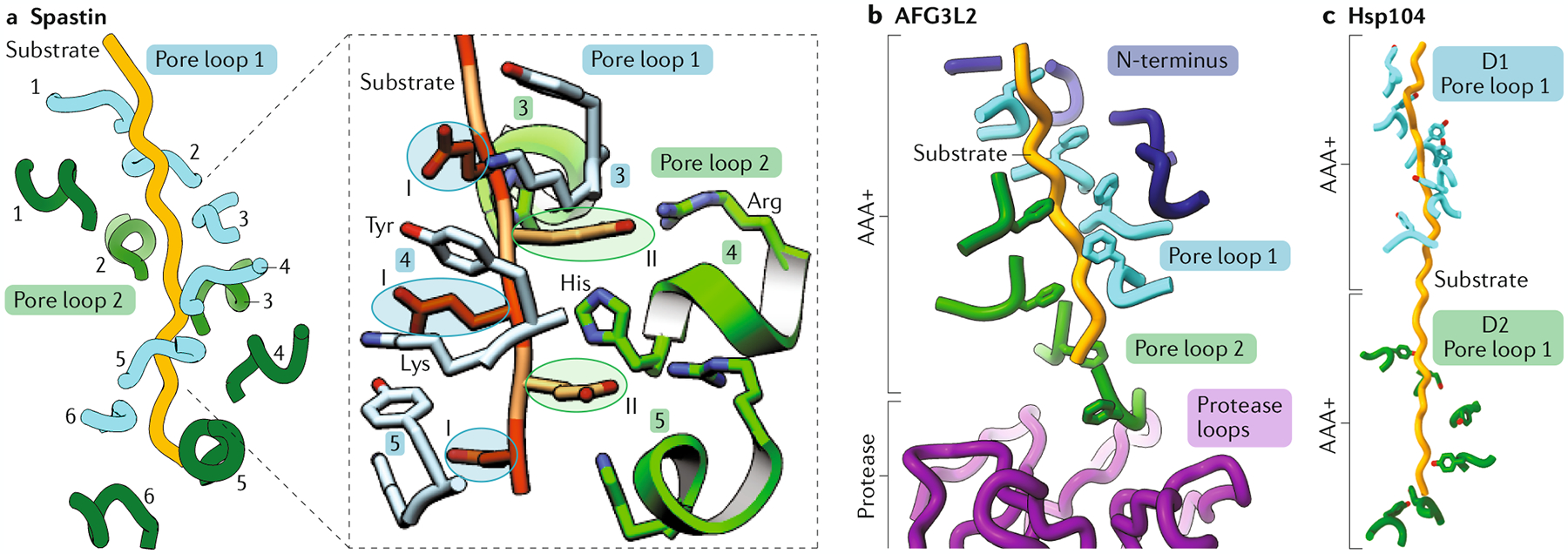
a | Pore loop 2 (green) of type I ATPase associated with diverse cellular activities (AAA+) protein spastin (pdb:6P07) assembles as a staircase parallel to the pore loop 1 (blue) staircase (in both cases, numbers indicate the subunit to which the pore loop belongs). The zoom shows the polyglutamate substrate threaded through the central pore of spastin. The residues of the substrate that insert into the class I pocket formed by pore loop 1 are circled in blue, whereas the residues that face the class II pocket are circled in green. The class I pocket includes the pore loop 1 aromatic (tyrosine, Tyr) and lysine (Lys) residues, both of which are shown in light blue. In the class II pocket, the positively charged residues (arginine (Arg) and histidine (His)) of the non-conserved pore loop 2 are shown in green. b | In AAA+ protease AFG3L2 (pdb:6NYY), pore loop 2 (green) and the amino terminus (N-terminus; dark blue) form spiral staircases additional to the one established by pore loop 1 that wrap around the substrate along the longitudinal axis of the complex. Pore loop 2 of AFG3L2 contains an insertion (Supplementary Fig. 1) that positions the pore loop 2 staircase low within the spiral, such that pore loop 2 of the lowest subunit of AFG3L2 protrudes deep into the proteolytic chamber (purple), contacting the centre of the protease ring. Pore loop 2 escorts the substrate across the degradation chamber, directly transferring it to the protease. c | Pore loop 1 of domain 1 (D1; blue) and domain 2 (D2; green) of the type II AAA+ protein Hsp104 (pdb:5VYA) assemble as individual spiral staircases that interact in tandem with the translocating substrate, thereby providing a cooperative grip on the substrate.
