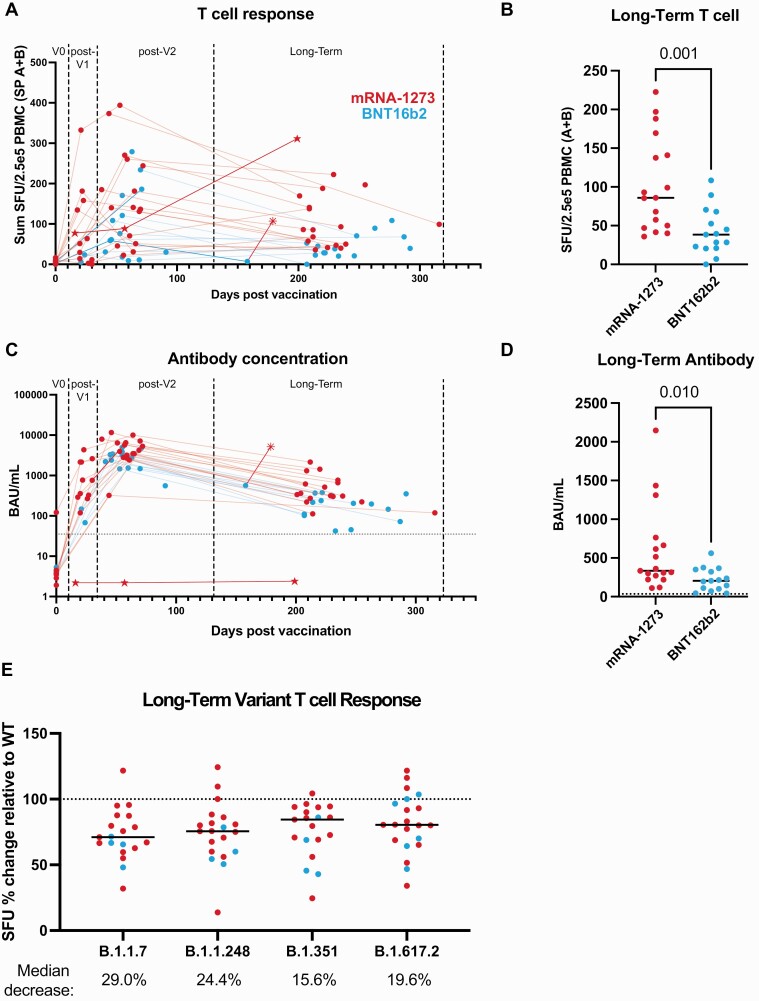
Figure 1.
BNT162b2 (Pfizer-BioNTech) has significantly lower T-cell and antibody responses compared with mRNA-1273 (Moderna) in a long-term follow-up postvaccination. A, T-cell responses from spike peptide pools (sum of the average SFU in response to pool A plus pool B) versus sample collection date in relation to first vaccine dose as evaluated by IFN-γ ELISpot. B, Comparison of long-term follow-up T-cell responses after vaccination with mRNA-1273 and BNT162b2. C, Quantified anti-spike IgG values versus sample collection date in relation to first vaccine dose, determined by ELISA. The dotted line represents the positivity cutoff of 35.2 BAU/mL. D, Comparison of mRNA-1273 and BNT162b2 anti-spike antibody values at long-term follow-up after vaccination. E, ELISpot response to variant peptide pools relative to the individual subject’s WT response at long-term follow-up after vaccination. The dotted line represents response to WT. Statistical comparisons were made using unpaired, 2-tailed Mann-Whitney test. Medians are shown as black lines: mRNA-1273 in red, BNT162b2 in blue; ☆ = rituximab-treated patient; * = received a third vaccination dose. Abbreviations: BAU, binding antibody units; ELISA, enzyme-linked immunosorbent assay; IFN-γ, interferon-gamma; IgG, immunoglobulin G; PBMC, peripheral blood mononuclear cells; SFU, spot-forming units; SP, spike pool; WT, wild-type.