Abstract
Beta (B.1.351)–variant coronavirus disease 2019 (COVID-19) disease was investigated in Qatar. Compared with the Alpha (B.1.1.7) variant, odds (95% confidence interval) of progressing to severe disease, critical disease, and COVID-19–related death were 1.24-fold (1.11–1.39), 1.49-fold (1.13–1.97), and 1.57-fold (1.03–2.43) higher, respectively, for the Beta variant.
Keywords: SARS-CoV-2, variant, infection, severe disease, epidemiology
Commencing in mid-January 2021, Qatar experienced a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Alpha [1] (B.1.1.7)–variant wave that peaked in the first week of March [2–5], but was immediately followed by a Beta [1] (B.1.351)–variant wave that peaked in the first week of April [2–6]. This created a unique epidemiologic situation that allowed comparative assessment of the severity, criticality, and fatality of these 2 variants.
METHODS
We investigated severity (acute-care hospitalization) [7], criticality (intensive care unit [ICU] hospitalization) [7], and fatality [8] of both variants through 8 case-control studies applied to the complete national cohorts of SARS-CoV-2 infections, coronavirus disease 2019 (COVID-19) disease cases, and COVID-19–related deaths in Qatar, a country with diverse demographics where 89% of the population comprises expatriates from over 150 countries [9]. Data on polymerase chain reaction (PCR) testing and clinical characteristics were extracted from the national federated COVID-19 databases that have captured all SARS-CoV-2–related data since the start of the epidemic. These databases were retrieved from the integrated nationwide digital-health information platform (universal healthcare system), and include all records of PCR testing, antibody testing, vaccinations, COVID-19 hospitalizations, infection severity classification, and COVID-19–related deaths. Databases are complete at the national level with no missing information.
Records of PCR testing and clinical data for hospitalized patients with COVID-19 were examined. Details of the laboratory methods for PCR testing are found in Supplementary Text 1. Each person who had a PCR-positive test result and hospital admission was subject to an infection-severity assessment every 3 days until discharge or death. Individuals who progressed to COVID-19 disease between the time of the PCR-positive test result and the end of the study were classified based on their worst outcome, starting with death [8], followed by critical disease [7], and then severe disease [7].
Cases in the case-control studies were persons who progressed to COVID-19 severe disease, critical disease, or death. Controls were persons with asymptomatic or mild SARS-CoV-2 infections. Cases and controls were matched at a ratio of 1:3 by 10-year age group, sex, and biweekly interval of the PCR diagnosis date. Every case in Qatar that met the inclusion criteria and that could be matched to a control was included in the study. Classification of case severity, criticality, and fatality followed the World Health Organization guidelines [7, 8], and assessments were made by trained medical personnel through individual chart reviews. Details of the COVID-19 severity, criticality, and fatality classification are found in Supplementary Text 2.
From 18 January until 15 February 2021, the Alpha-variant wave expanded rapidly and weekly rounds of viral genome sequencing [2–5] of randomly collected samples confirmed the presence of this and other originally circulating “wild-type” variants, but documented only limited presence of the Beta variant and no other variants of concern [2–5]. This allowed a comparative assessment for the Alpha variant versus wild-type variants during this specific time frame (Supplementary Text 3). From 8 March through 31 May 2021, the Beta-variant wave expanded rapidly and viral genome sequencing [2–5] and multiplex quantitative reverse-transcription PCR (RT-qPCR) variant screening [2–6] indicated dominance of the Beta and Alpha variants, with limited presence of other variants [2–6]. This enabled comparisons between the Beta versus Alpha variants during this specific time frame (Supplementary Text 3). The Delta [1] (B.1.617.2) variant has been introduced more recently in Qatar, and it remains at a low incidence as of 11 July 2021 [4–6]. Further details on the classification of infections by variant type are found in Supplementary Text 3.
Descriptive statistics (frequency distributions and measures of central tendency) were used to characterize the study samples. Two-sided P values ofless than .05 were considered statistically significant. Odds ratios and their associated 95% confidence intervals (CIs) were calculated using the exact method. Confidence intervals were not adjusted for multiplicity. Interactions were not investigated. Two sensitivity analyses were conducted by first adjusting for age, and second by adjusting for age and sex, in logistic regression analyses. Statistical analyses were conducted in STATA/SE version 17.0 (StataCorp).
The study was approved by the Hamad Medical Corporation and Weill Cornell Medicine–Qatar Institutional Review Boards with waiver of informed consent. Reporting of the study followed the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines (Supplementary Table 1).
RESULTS
Demographic characteristics of the samples for each disease outcome in assessing the severity, criticality, and fatality of the Alpha variant compared with the wild-type variants are presented in Supplementary Table 2. Compared with wild-type variants, the odds of progressing to severe disease were 1.48-fold (95% CI: 1.18–1.84-fold) higher for the Alpha variant (Table 1). The odds of progressing to critical disease were 1.58-fold (95% CI: .79–3.10-fold) higher, but did not reach statistical significance, perhaps because of the small number of critical disease cases. There were also too few COVID-19–related deaths to assess the fatality of the Alpha variant.
Table 1.
Infection Severity, Criticality, and Fatality of the Alpha and Beta Variants in the Population of Qatar
| Groups | Infection Severitya | Assessment of Severity, Criticality, and Fatality of the Alpha Variant Compared With the Wild-type Variants Circulating Between 18 January and 15 February 2021b | Assessment of Severity, Criticality, and Fatality of the Beta Variant Compared With the Alpha Variant Between 8 March and 31 May 2021c | ||||
|---|---|---|---|---|---|---|---|
| Infection With an Alpha Variant | Infection With a Wild-type Variant | Odds Ratio (95% CI) | Infection With a Beta Variant | Infection With an Alpha Variant | Odds Ratio (95% CI) | ||
| Cases | Severe disease | 188 | 279 | 1.48 (1.18–1.84) | 2036 | 483 | 1.24 (1.11–1.39) |
| Controls | Asymptomatic or mild infection | 431 | 944 | 5806 | 1707 | ||
| Cases | Critical disease | 21 | 37 | 1.58 (.79–3.10) | 382 | 81 | 1.49 (1.13–1.97) |
| Controls | Asymptomatic or mild infection | 49 | 125 | 1056 | 333 | ||
| Cases | Severe or critical disease | 209 | 316 | 1.45 (1.18–1.79) | 2418 | 564 | 1.28 (1.15–1.42) |
| Controls | Asymptomatic or mild infection | 480 | 1054 | 6764 | 2019 | ||
| Cases | COVID-19–related death | 2 | 9 | .83 (.07–5.58) | 142 | 37 | 1.57 (1.03–2.43) |
| Controls | Asymptomatic or mild infection | 7 | 26 | 381 | 156 | ||
Cases and controls were matched on a ratio of 1:3 by 10-year age group, sex, and biweekly interval of the PCR diagnosis date.
Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019; PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Severe disease, critical disease, and COVID-19–related death were defined based on the World Health Organization criteria for classifying SARS-CoV-2 infection severity [7] and COVID-19–related death [8].
From 18 January to 15 February 2021, the Alpha variant and other wild-type variants dominated incidence, with limited presence of the Beta variant [2–5].
From 8 March to 31 May 2021, the Beta and Alpha variants dominated incidence, with limited presence of other variants [2–6].
The median time from PCR-positive test to severe disease was 7 days (mean: 7 days; interquartile range [IQR]: 4–9 days) for Alpha cases and 6 days (mean: 6 days; IQR: 3–8 days) for wild-type cases. The median time from severe to critical disease was 4 days (mean: 5 days; IQR: 3–7 days) for Alpha cases and 4 days (mean: 6 days; IQR: 3–7 days) for wild-type cases. There were too few deaths in this analysis to provide summary statistics.
Demographic characteristics of the samples for each disease outcome in assessing the severity, criticality, and fatality of the Beta variant compared with the Alpha variant are presented in Supplementary Table 3. Compared with the Alpha variant, the odds of progressing to severe disease were 1.24-fold (95% CI: 1.11–1.39-fold) higher for the Beta variant (Table 1). The odds of progressing to critical disease were 1.49-fold (95% CI: 1.13–1.97-fold) higher, and the odds of COVID-19–related death were 1.57-fold (95% CI: 1.03–2.43-fold) higher.
The median time from PCR-positive test to severe disease was 5 days (mean: 5 days; IQR: 3–8 days) for Beta cases and 5 days (mean: 6 days; IQR: 3–8 days) for Alpha cases. The median time from severe to critical disease was 7 days (mean: 9 days; IQR: 4–11 days) for Beta cases and 7 days (mean: 10 days; IQR: 4–14 days) for Alpha cases. The median time from critical disease to death was 15 days (mean: 20 days; IQR: 6–26 days) for Beta cases and 15 days (mean: 21 days; IQR: 6–32 days) for Alpha cases.
Sensitivity analyses confirmed the above results (Supplementary Table 4).
DISCUSSION
The Alpha variant presented a 48% higher risk of severe disease than wild-type variants in the population of Qatar, affirming its greater gravity [10, 11] (odds ratio approximates risk ratio for rare outcomes). Infection with the Beta variant was associated with even greater risks of severe and critical disease and COVID-19–related death, affirming earlier observational analyses suggesting its high gravity [11, 12]. Compared with the Alpha variant, infections with the Beta variant posed a 24% higher risk of severe disease, 49% higher risk of critical disease, and 57% higher risk of COVID-19–related death.
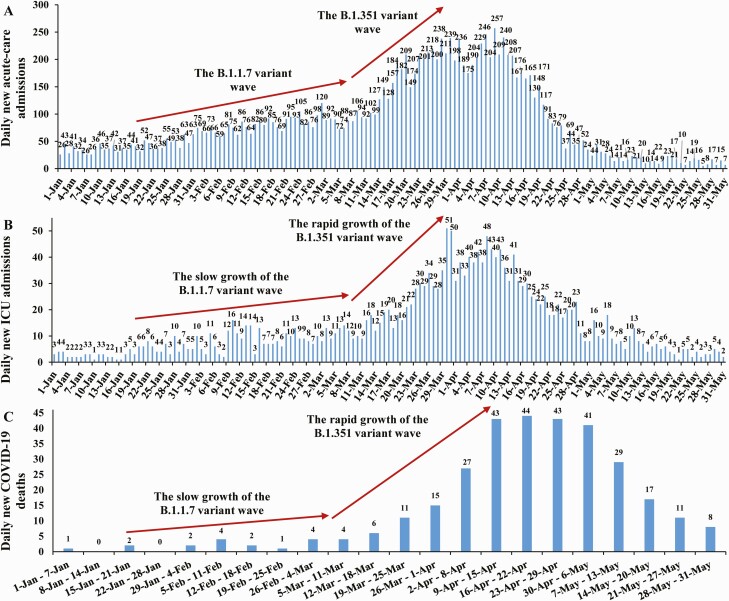
These results explain the changing pattern of hospitalizations and deaths seen during the Beta wave compared with the Alpha wave (Figure 1). Acute-care admissions doubled during the Beta wave, but ICU admissions and deaths quadrupled, with the disproportionally greater effect of this variant on critical disease and COVID-19–related death.
Figure 1.
Number of (A) daily new COVID-19 acute-care hospital admissions, (B) daily new COVID-19 ICU hospital admissions, and (C) COVID-19–related deaths in Qatar. Abbreviations: COVID-19, coronavirus disease 2019; ICU, intensive care unit.
Limitations include the smaller sample sizes of critical disease and COVID-19–related deaths in the Alpha-variant analysis (Supplementary Table 2) compared with the Beta-variant analysis (Supplementary Table 3), as COVID-19 criticality and fatality have been low in Qatar’s predominantly young and working-age population [9, 13], leading to statistically nonsignificant results and wider 95% CIs. Data on comorbid conditions were not available to study investigators; hence, they could not be explicitly factored in our analysis. Nevertheless, matching and adjusting for age in analysis may have served as a proxy, given that comorbidities are associated with old age. Furthermore, with the young population structure [9], we anticipate that only a small proportion of the study population may have had serious comorbid conditions. However, our findings may not be entirely generalizable to other settings, where elderly people constitute a sizable proportion of the population. Imperfect assay sensitivity and specificity of PCR testing may have affected infection ascertainment. However, all PCR testing was performed with extensively used, investigated, and validated commercial platforms having essentially 100% sensitivity and specificity (Supplementary Text 1). Unlike blinded randomized clinical trials, the investigated observational cohorts were neither blinded nor randomized.
In conclusion, the Alpha variant is associated with a 48% higher risk of severe disease than wild-type variants. In turn, the Beta variant is associated with a 24% higher risk of severe disease than the Alpha variant, and strikingly, an even higher risk of critical disease (49%) and COVID-19–related death (57%). These findings highlight risks to healthcare systems, particularly intensive care facilities and resources, in the event of a globally increased circulation of the Beta variant. With the Delta variant increasingly dominating incidence in Qatar and other countries, an extension of this work could be a comparison of disease outcomes of Delta versus Alpha and Beta infections.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. L. J. A. conceived and co-designed the study, led the statistical analyses, and co-wrote the first draft of the article. H. C. co-designed the study, performed the statistical analyses, and co-wrote the first draft of the article. All authors contributed to data collection and acquisition, database development, discussion and interpretation of the results, and to the writing of the manuscript. All authors have read and approved the final manuscript.
Acknowledgments. The authors acknowledge the many dedicated individuals at Hamad Medical Corporation, the Ministry of Public Health, the Primary Health Care Corporation, the Qatar Biobank, and Sidra Medicine for their diligent efforts and contributions to make this study possible. The dataset of this study is a property of the Qatar Ministry of Public Health that was provided to the researchers through a restricted-access agreement that prevents sharing the dataset with a third party or publicly. Aggregate data are available within the manuscript and its Supplementary Material. A limited dataset including the cases and controls and their associated variables that were used in the analysis can be made available for researchers upon request to the corresponding author of this study.
Disclaimer. The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the article. Statements made herein are solely the responsibility of the authors.
Financial support. This work was supported by the Biomedical Research Program and the Biostatistics, Epidemiology, and Biomathematics Research Core, both at Weill Cornell Medicine–Qatar, as well as support provided by the Ministry of Public Health and Hamad Medical Corporation. The Qatar Genome Programme supported the viral genome sequencing.
Potential conflicts of interest. A. A. B. has received institutional grant funding from Gilead Sciences unrelated to the work presented in this paper. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Laith J Abu-Raddad, Infectious Disease Epidemiology Group, Weill Cornell Medicine–Qatar, Cornell University, Qatar Foundation—Education City, Doha, Qatar; World Health Organization Collaborating Centre for Disease Epidemiology Analytics on HIV/AIDS, Sexually Transmitted Infections, and Viral Hepatitis, Weill Cornell Medicine–Qatar, Cornell University, Qatar Foundation—Education City, Doha, Qatar; Department of Population Health Sciences, Weill Cornell Medicine, Cornell University, New York, New York, USA; Department of Public Health, College of Health Sciences, Member of QU Health, Qatar University, Doha, Qatar.
Hiam Chemaitelly, Infectious Disease Epidemiology Group, Weill Cornell Medicine–Qatar, Cornell University, Qatar Foundation—Education City, Doha, Qatar; World Health Organization Collaborating Centre for Disease Epidemiology Analytics on HIV/AIDS, Sexually Transmitted Infections, and Viral Hepatitis, Weill Cornell Medicine–Qatar, Cornell University, Qatar Foundation—Education City, Doha, Qatar.
Houssein H Ayoub, Mathematics Program, Department of Mathematics, Statistics, and Physics, College of Arts and Sciences, Qatar University, Doha, Qatar.
Hadi M Yassine, Biomedical Research Center, Member of QU Health, Qatar University, Doha, Qatar; Department of Biomedical Science, College of Health Sciences, Member of QU Health, Qatar University, Doha, Qatar.
Fatiha M Benslimane, Biomedical Research Center, Member of QU Health, Qatar University, Doha, Qatar; Department of Biomedical Science, College of Health Sciences, Member of QU Health, Qatar University, Doha, Qatar.
Hebah A Al Khatib, Biomedical Research Center, Member of QU Health, Qatar University, Doha, Qatar; Department of Biomedical Science, College of Health Sciences, Member of QU Health, Qatar University, Doha, Qatar.
Patrick Tang, Department of Pathology, Sidra Medicine, Doha, Qatar.
Mohammad R Hasan, Department of Pathology, Sidra Medicine, Doha, Qatar.
Peter Coyle, Biomedical Research Center, Member of QU Health, Qatar University, Doha, Qatar; Hamad Medical Corporation, Doha, Qatar; Wellcome-Wolfson Institute for Experimental Medicine, Queens University, Belfast, United Kingdom.
Sawsan AlMukdad, Infectious Disease Epidemiology Group, Weill Cornell Medicine–Qatar, Cornell University, Qatar Foundation—Education City, Doha, Qatar; World Health Organization Collaborating Centre for Disease Epidemiology Analytics on HIV/AIDS, Sexually Transmitted Infections, and Viral Hepatitis, Weill Cornell Medicine–Qatar, Cornell University, Qatar Foundation—Education City, Doha, Qatar.
Zaina Al Kanaani, Hamad Medical Corporation, Doha, Qatar.
Einas Al Kuwari, Hamad Medical Corporation, Doha, Qatar.
Andrew Jeremijenko, Hamad Medical Corporation, Doha, Qatar.
Anvar Hassan Kaleeckal, Hamad Medical Corporation, Doha, Qatar.
Ali Nizar Latif, Hamad Medical Corporation, Doha, Qatar.
Riyazuddin Mohammad Shaik, Hamad Medical Corporation, Doha, Qatar.
Hanan F Abdul Rahim, Department of Public Health, College of Health Sciences, Member of QU Health, Qatar University, Doha, Qatar.
Gheyath K Nasrallah, Biomedical Research Center, Member of QU Health, Qatar University, Doha, Qatar; Department of Biomedical Science, College of Health Sciences, Member of QU Health, Qatar University, Doha, Qatar.
Mohamed Ghaith Al Kuwari, Primary Health Care Corporation, Doha, Qatarand.
Adeel A Butt, Department of Population Health Sciences, Weill Cornell Medicine, Cornell University, New York, New York, USA; Hamad Medical Corporation, Doha, Qatar.
Hamad Eid Al Romaihi, Ministry of Public Health, Doha, Qatar.
Mohamed H Al-Thani, Ministry of Public Health, Doha, Qatar.
Abdullatif Al Khal, Hamad Medical Corporation, Doha, Qatar.
Roberto Bertollini, Ministry of Public Health, Doha, Qatar.
References
- 1. World Health Organization. Tracking SARS-CoV-2 variants. 2021. Available at: https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/. Accessed 5 June 2021.
- 2. Abu-Raddad LJ, Chemaitelly H, Butt AA; National Study Group for Covid-19 Vaccination. . Effectiveness of the BNT162b2 Covid-19 vaccine against the B.1.1.7 and B.1.351 variants. N Engl J Med 2021; 385:187–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chemaitelly H, Yassine HM, Benslimane FM, et al. . mRNA-1273 COVID-19 vaccine effectiveness against the B.1.1.7 and B.1.351 variants and severe COVID-19 disease in Qatar. Nat Med 2021; 27:1614–21. [DOI] [PubMed] [Google Scholar]
- 4. National Project of Surveillance for Variants of Concern and Viral Genome Sequencing. Qatar viral genome sequencing data. Data on randomly collected samples. Available at: https://www.gisaid.org/phylodynamics/global/nextstrain/. Accessed 11 October 2021.
- 5. Benslimane FM, Al Khatib HA, Al-Jamal O, et al. . One year of SARS-CoV-2: genomic characterization of COVID-19 outbreak in Qatar. medRxiv [Preprint]. May 20, 2021. Available at: https://www.medrxiv.org/content/10.1101/2021.05.19.21257433v2. Accessed 11 October 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hasan MR, Kalikiri MKR, Mirza F, et al. ; National Study Group for COVID-19 Epidemiology in Qatar. . Real-time SARS-CoV-2 genotyping by high-throughput multiplex PCR reveals the epidemiology of the variants of concern in Qatar. Int J Infect Dis 2021; 112:52–4. [DOI] [PubMed] [Google Scholar]
- 7. World Health Organization. COVID-19 clinical management: living guidance. 2021. Available at: https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-1. Accessed 15 May 2021.
- 8. World Health Organization. International guidelines for certification and classification (coding) of COVID-19 as cause of death. 2021. Available at: https://www.who.int/classifications/icd/Guidelines_Cause_of_Death_COVID-19-20200420-EN.pdf?ua=1.DocumentNumber:WHO/HQ/DDI/DNA/CAT. Accessed 31 May 2021.
- 9. Abu-Raddad LJ, Chemaitelly H, Ayoub HH, et al. . Characterizing the Qatar advanced-phase SARS-CoV-2 epidemic. Sci Rep 2021; 11:6233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Challen R, Brooks-Pollock E, Read JM, Dyson L, Tsaneva-Atanasova K, Danon L.. Risk of mortality in patients infected with SARS-CoV-2 variant of concern 202012/1: matched cohort study. BMJ 2021; 372:n579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Funk T, Pharris A, Spiteri G, et al. . Characteristics of SARS-CoV-2 variants of concern B.1.1.7, B.1.351 or P.1: data from seven EU/EEA countries, weeks 38/2020 to 10/2021. Euro Surveill 2021; 26:2100348. doi: 10.2807/1560-7917.ES.2021.26.16.2100348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jassat W, Mudara C, Ozougwu L, et al. ; DATCOV Author Group. . Difference in mortality among individuals admitted to hospital with COVID-19 during the first and second waves in South Africa: a cohort study. Lancet Glob Health 2021; 9:e1216–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Seedat S, Chemaitelly H, Ayoub HH, et al. . SARS-CoV-2 infection hospitalization, severity, criticality, and fatality rates in Qatar. Sci Rep 2021; 11:18182. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.