Figure 5. cDC1 accumulation requires innate lymphoid support.
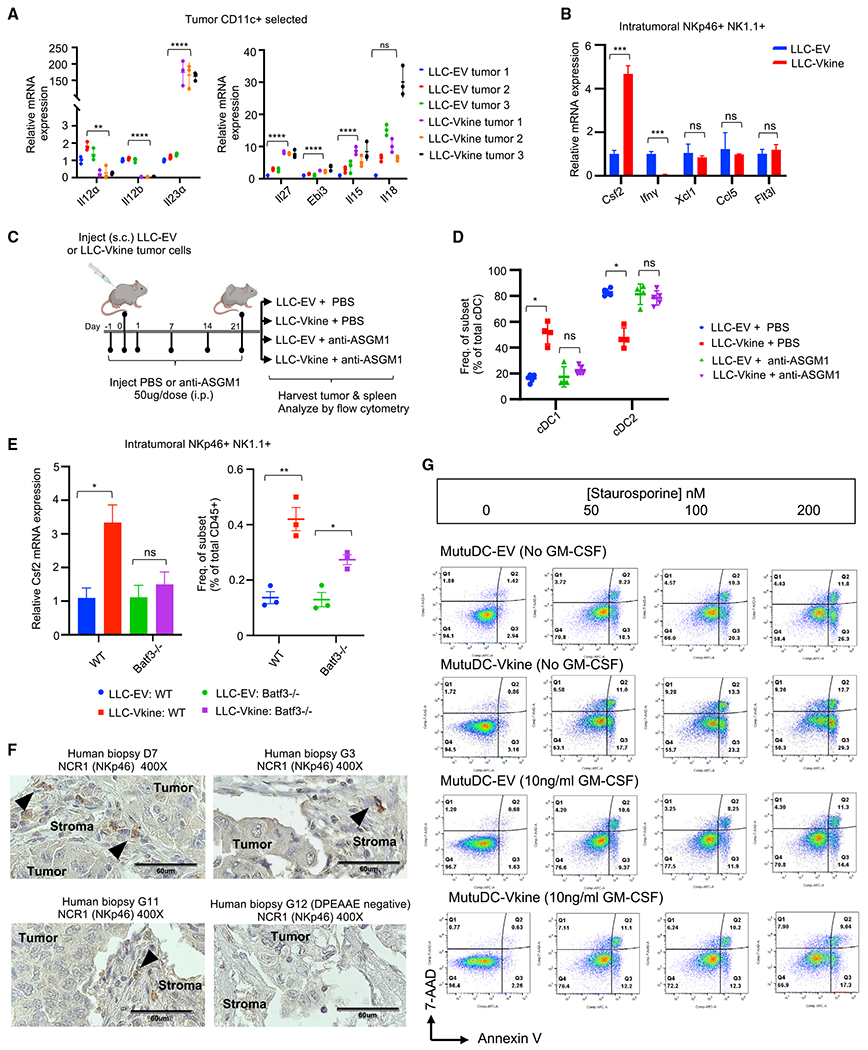
(A) RT-PCR for NK cell-activating cytokine transcripts expressed by ex vivo magnetically separated CD11c+ cells from LLC-EV and LLC-Vkine tumors.
(B) RT-PCR profile of NKp46+ NK1.1+ cells flow-sorted from LLC-EV and LLC-Vkine tumors. Data are presented as mean ± SEM, n = 3 for each group.
(C) Schematic of the NK cell depletion experiment.
(D) Summary of cDC subset frequency by flow cytometric analysis in LLC-EV versus LLC-Vkine tumors after treatment with NK cell-depleting antibody (anti-ASGM1) or vehicle (PBS).
(E) Csf2 (GM-CSF) RT-PCR of RNA extracted from NKp46+ NK1.1+ cells flow-sorted from LLC-EV and LLC-Vkine tumors growing in WT or Batf3−/− hosts.
(F) Stromal localization of NCR1+ (NKp46+) cells in human lung cancers (chromogen, DAB; counterstain, hematoxylin). 40× objective: scale bar, 60 μm.
(G) Annexin V/7-AAD apoptosis assay of MutuDC1940-EV or -Vkine dendritic cells exposed to graded staurosporine concentrations with or without murine GM-CSF.
Data are presented as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. In vitro experiments were performed in technical triplicates. In vivo cohort sizes are shown in individual panels. All experiments were reproduced independently at least twice.
