Figure 6. Stroma-licensed cDC1s are “poised” and hypersensitive to nucleic acid sensing in vivo.
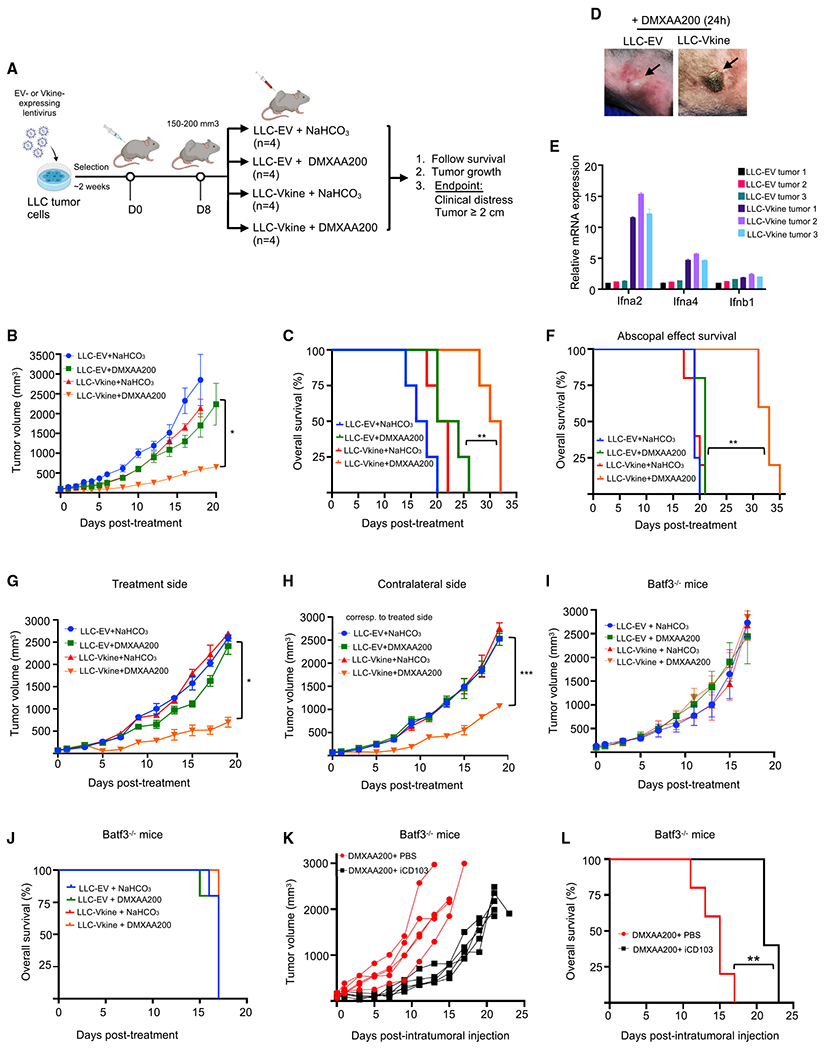
(A) Schematic of the experiment.
(B) Growth curves of LLC-EV and LLC-Vkine tumors challenged with a single subtherapeutic dose (200 μg) of intratumoral (IT) DMXAA (DMXAA200) or vehicle (NaHCO3) on day 0.
(C) Kaplan-Meier survival curves for the experiment in (B); **p < 0.01 by log rank test.
(D) Representative images showing development of hemorrhagic necrosis and a necrotic eschar in LLC-Vkine but not LLC-EV tumors 24 h after IT DMXAA200 administration.
(E) Transcriptomic profile of LLC-EV and LLC-Vkine tumors harvested 2 h after IT DMXAA200 (Table S6).
(F) Versikine -DMXAA synergy generates an abscopal effect in LLC tumors that produces a survival advantage. **p < 0.01 by log rank test.
(G) Growth curves of treatment-side LLC-EV and LLC-Vkine tumors challenged with a single subtherapeutic dose (200 μg) of IT DMXAA (DMXAA200) or vehicle (NaHCO3) on day 0.
(H) Growth curves of contralateral side unmanipulated LLC tumors; treated side as in (G).
(I) Response to DMXAA200 is lost in Batf3−/− recipients. Shown are growth curves of LLC-EV and LLC-Vkine tumors challenged with a single subtherapeutic dose (200 μg) of IT DMXAA (DMXAA200) or vehicle (NaHCO3) on day 0 in Batf3−/− recipients.
(J) Batf3 loss abrogates the survival advantage seen in the WT (C).
(K) Efficacy of DMXAA200 in LLC-Vkine tumors implanted into Batf3−/− recipients is restored after adoptive transfer of iCD103 (Figures S6D and S6E).
(L) Adoptive transfer of iCD103 in LLC-Vkine tumors implanted into Batf3−/− recipients restores the survival advantage of mice treated with DMXAA200. **p < 0.01 by log rank test.
Data are presented as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. In vitro experiments were performed in technical triplicates. In vivo cohort sizes are shown in individual panels. All experiments were reproduced independently at least twice.
