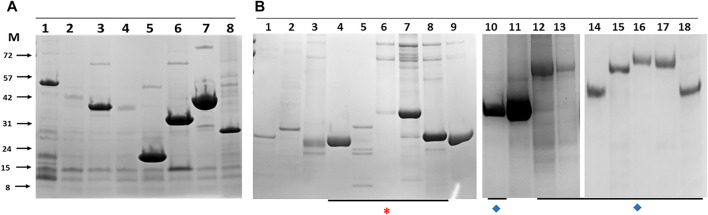
FIGURE 3.
Example histidine-tagged M. bovis proteins purified by immobilised metal affinity chromatography pull down with magnetic beads from E.coli (A) and insect cells (B). The lane identity and predicted molecular masses are: Panel A, 1-Mb0704 (44 kDa), 2-Mb3070 (37 kDa), 3-Mb2054 (36 kDa), 4-Mb2002_Mb0062 (35 kDa), 5-Mb0671 (17 kDa), 6-Mb0854 (31 kDa), 7- Mb3157c_Mb3743c (37 kDa), 8-Mb0337 (26 kDa). Panel B, 1-PE5_PE32 (19.5 kDa), 2-LpqN (25 kDa), 3-LpqX (22 kDa), 4-EsxAB (21.2 kDa), 5-EsxFDE (27.3 kDa), 6-EsxNOQ (30 kDa), 7- EsxRST (32 kDa), 8-EsxU (24 kDa), 9- Mb0485 (21 kDa), 10-PPE41_PE25 (34 kDa), 11-Mb1228 (39 kDa), 12-PPE69_PE36 (46.6 kDa), 13- PPE68_PE35 (47.5 kDa), 14-PPE14_PE7 (51 kDa), 15-PPE4_PE5 (67 kDa), 16- PPE15_PE8 (66 kDa), 17- PPE20_PE15 (67 kDa), 18-PPE31_PE20 (51 kDa). Asterisk (*) indicates all Esx related proteins, most as concatenates. Diamonds (♦) indicate concatenated PPE-PE pairs. M indicates the marker track, the molecular masses of which are given on the left of panel (A) in kilodaltons.