Abstract
Objective
To determine the cost-effectiveness of Xpert Omni compared with Xpert MTB/Rif for point-of-care diagnosis of tuberculosis among presumptive cases in a low-resource, high burden facility.
Design
Cost-effectiveness analysis from the provider’s perspective.
Setting
A low-resource, high tuberculosis burden district in Eastern Uganda.
Participants
A provider’s perspective was used, and thus, data were collected from experts in the field of tuberculosis diagnosis purposively selected at the local, subnational and national levels.
Methods
A decision analysis model was contracted from TreeAge comparing Xpert MTB/Rif and Xpert Omni. Cost estimation was done using the ingredients’ approach. One-way deterministic sensitivity analyses were performed to identify the most influential model parameters.
Outcome measure
The outcome measure was incremental cost per additional test diagnosed expressed as the incremental cost-effectiveness ratio.
Results
The total cost per test for Xpert MTB/Rif was US$14.933. Cartridge and reagent kits contributed to 67% of Xpert MTB/Rif costs. Sample transport costs increased the cost per test of Xpert MTB/Rif by $1.28. The total cost per test for Xpert Omni was $16.153. Cartridge and reagent kits contributed to over 71.2% of Xpert Omni’s cost per test. The incremental cost-effectiveness ratio for using Xpert Omni as a replacement for Xpert MTB/Rif was US$30.73 per additional case detected. There was no dominance noted in the cost-effectiveness analysis, meaning no strategy was dominant over the other.
Conclusion
The use of Xpert Omni at the point-of-care health facility was more effective but with an increased cost compared with Xpert MTB/Rif at the centralised referral testing facility.
Keywords: health economics, epidemiology, public health, health policy
Strengths and limitations of this study.
Nationally representative data sources for prevalence based on a particular population were used to increase generalisability.
Sensitivity analysis was done to determine the uncertainty in the estimates.
The costing was solely based on the provider’s perspective and does not contribute to the WHO End TB strategy goal to reduce catastrophic costs for patients with tuberculosis.
Treatment outcomes as measures of cost-effectiveness were not measured.
Introduction
Tuberculosis (TB) remains a global epidemic infecting approximately one-third of the world’s population. WHO reported an estimated 10 million incident TB cases in 2020 and 1.3 million TB deaths among HIV-negative people with an additional 214 000 deaths among HIV people.1 Uganda falls among the world’s top 16 countries that contribute 93% of the world’s TB burden and ranks among the top 12 countries with the highest HIV–TB coinfection globally.1 Uganda has an estimated TB prevalence of 33.7% of TB among HIV-positive people. The prevalence is almost half (46.3%) among HIV-positive men compared with 25.2% among HIV-positive women.2 3 TB case detection rate in Uganda is low.4 The Uganda National Health Sector Performance report5 indicated the detection rate of all forms of TB was estimated at 52% with a treatment success rate of 54% and a death rate of 5.4% in all diagnosed cases.
Insufficient case detection is a persistent obstacle to furthering the WHO’s stated goal of eliminating TB by 2035.6 One strategy for achieving this goal is to enhance TB case finding by implementing new diagnostic tests with improved sensitivity and turnaround time, particularly in settings that primarily rely on sputum smear microscopy for diagnostics.7 Although the introduction of Xpert MTB/Rif molecular assay for the detection of TB and resistance to rifampicin has provided substantial improvements in sensitivity over sputum smear microscopy.8 It is reported to give a false-positive result for strains that carry phenotypically silent mutations. It has decreased capacity to detect rpoBC533G mutations responsible for some cases of RIF-R9–11 and occasionally gives false-positive RIF-R results for especially paucibacillary samples.12 In addition, no evidence to date proves that Xpert MTB/Rif has provided a substantial clinical impact in reducing TB morbidity and mortality.13 14
In 2015, Cepheid unveiled the GeneXpert Omni, (referred to us as Xpert Omni in this study), which is the world’s most portable point-of-care (POC) diagnostic test. Xpert Omni runs the same high-quality PCR-based cartridge tests as Cepheid’s Xpert MTB/Rif Systems. It can detect TB with concentrations as low as 130 bacilli/mL of sputum. The Xpert Omni will provide increased access to rapid TB testing in remote areas with unstable electricity supply.15 It uses an Xpert Ultra cartridge whose performance has been subsequently evaluated in a large 10-site and eight-country study that confirmed its increased sensitivity for the diagnosis of active TB relative to the existing standard Xpert MTB/Rif cartridge.16 A multicentre non-inferiority study at 10 sites in eight low-income and middle-income countries demonstrated Xpert Omni Ultra cartridge’s general sensitivity was 5% higher than standard Xpert MTB/Rif cartridge, 17% higher in smear-negative samples and 12% higher in HIV-infected patients. However, its general specificity was up to 3% lower than the standard cartridge.16 Based on its improved sensitivity, the WHO endorsed the Ultra cartridge for use in all settings.17
To enhance TB case detection, the Ministry of Health in Uganda has scaled up the use of Gene Xpert MTB/Rif as a standard of care for TB diagnosis at hub laboratories. In Serere district, GeneXpert testing is centralised at the hub laboratory located in Serere Health Center IV. Peripheral facilities refer smear-negative samples to this hub. There is still inadequate capacity to provide laboratory confirmation of TB by Xpert MTB/Rif at POC in lower facilities due to the complexity of its technology and high costs. This is contributing to gaps in finding, treating and following up on patients with TB in remote peripheral facilities.
GeneXpert Omni is user-friendly compared with the standard of care test and maybe a better POC PCR test to be used at the peripheral facilities that are ordinarily transporting samples to the centralised testing laboratory hub. However, there is no certainty that the improved sensitivity of Xpert Omni is worth the cost when applied as a POC test. As to whether the increased sensitivity of Xpert Omni is worth the cost is not known since no such studies have been done in a similar setting. We thus set out to establish the cost-effectiveness of Xpert MTB/Rif compared with Xpert Omni for its use at POC in a high-burden, low-resource setting in a remote district in Eastern Uganda.
Methods
Study site and design
The study was done in Pingire Health Center III, Serere district in Eastern Uganda. This facility serves the fishing communities of Lake Kyoga with demonstrated high prevalence of HIV, a risk factor for TB.18 We used a decision tree to support cost-effect analysis. The analysis was done from the providers’ perspective. The providers included the Ministry of Health, donor agencies and implementing partners. The reference case used in the cost-effect analysis was an HIV-positive male adult presumptive TB case above 35 years without previous TB. This case was chosen because the incidence of TB in Uganda among this population is higher than in all other populations. It is a population with poor health-seeking behaviour that would benefit more from the POC diagnosis.3 The time horizon for cost-effectiveness was about 1 month, which includes the time from identification of the presumptive cases to the final determination of TB status by either method. The model did not include the long-term effects of missed diagnoses and delays in treatment.
Description of study alternatives
The comparator was the standard of care (Xpert MTB/Rif), which consists of the instrument, a computer and a barcode scanner and requires single-use disposable Xpert MTB/Rif cartridges that contain assay reagents. This is a four-module cartridge-based nucleic acid amplification test automated to detect MTB DNA and RR. The Xpert MTB/Rif detects DNA sequences specific for MTB and RR by PCR. The primers in the Xpert MTB/Rif assay amplify a portion of the rpoB gene containing the eight base pair ‘core’ region. The probes can differentiate between the conserved wild-type sequence and mutations in the core region that is associated with resistance to RIF.19 The Xpert MTB/Rif purifies and concentrates TB bacilli from sputum samples, isolates genomic material from the captured bacteria by sonication and subsequently amplifies the genomic DNA by PCR. The process identifies all the clinically relevant Rifampicin Resistant-inducing mutations in the RNA polymerase beta (rpoB) gene in the Mycobacterium tuberculosis genome in a real-time format using fluorescent probes called molecular beacons.20 The steps involved in processing the sample, amplification and detection of the mycobacterial DNA are automated. This enables reporting of test results in 2–3 hours.21 The Xpert MTB/Rif machine is located at the hub laboratory, which serves the entire district and lower facilities refer samples through the sample referral system facilitated by riders.22
The intervention was the Xpert Omni. Xpert Omni is a single-module battery-powered platform whose mechanism of operation is the same as the Xpert MTB/Rif. The Xpert Omni platform operates on a unique cartridge with a near-field communication (NFC) chip for connectivity and data transfer. The device is small and portable, durable, battery-operated, wireless and web enabled, allowing instrument and test information to be transmitted in real time. Its cartridge technology uses advanced fluidics that regulates the testing process within the cartridge from nucleic acid extraction to amplification to detection. The intuitive user interface is driven by a dedicated mobile that controls a single module. Secure cloud-based connectivity integrates real-time data streams for greater productivity and performance. The mobile device to a single module enables the placement of the device in all testing environments.15 The device provides increased access to rapid, accurate and potentially life-saving TB testing in some of the most remote areas. Due to the portable nature of the device and it being battery powered, the testing can be done at lower health facilities without electricity supply as opposed to transporting TB samples to a centralised testing laboratory hub.
Cost of data collection
Cost estimates of Xpert MTB/Rif and Xpert Omni machines, accessories, cartridges, calibration and warranty were obtained from the negotiated prices provided for low-income countries.23 Additional information was obtained from budgetary documentation reviews, procurement guides and publicly available product information. Expert opinion about the cost of inputs was sought from suppliers, implementing partners, district health officials, laboratory personnel and sample transporters. Previous costing studies in Uganda were reviewed to validate these estimates.22 24 Costs associated with sample collection, biosafety requirements and transportation to the hub were estimated from Pingire Health Center III and Serere Health Center IV. More cost data were obtained from the literature, national medical stores’ price catalogue,25 joint medical stores’ price catalogue26 and health facility records such as delivery notes, budgets and invoices. Cost data for Xpert Omni was mostly got from literature (table 1) and expert opinion from the National TB Reference Laboratory in Kampala because the machine was not available at the study site.
Table 1.
Decision model parameters
| Parameter | Base case value (%) | Low (%) | High (%) | Source/reference |
| Prevalence of TB among HIV-positive patients. | 20 | 10 | 30 | 3, 40 |
| Xpert MTB/Rif (standard cartridge) | ||||
| Sensitivity | 77 | 68 | 84 | 16, 33, 30 |
| Specificity | 98 | 97 | 99 | 16, 31, 30 |
| Cost of Xpert MTB/Rif | 14.924 | 12.547 | 17.301 | Primary cost data |
| Xpert Omni (Ultra cartridge) | ||||
| Sensitivity | 90 | 83 | 95 | 16, 36 |
| Specificity | 96 | 94 | 98 | 16, 36 |
| Cost of Xpert Omni | 16.153 | 13.58 | 18.726 | Primary cost data |
TB, tuberculosis.
Costing inputs
Costs for Xpert MTB/Rif included: Xpert MTB/Rif four-module machine, power back-up, printer, installation and building space. Other costs for Xpert MTB/Rif include standard cartridge and reagent kit, staff salary, sample transport costs, calibration costs, maintenance, staff training costs, utilities and consumables. Costs for Xpert Omni included: Xpert Omni machine and accessories; installation costs; supplemental rechargeable power battery and iPhone 5; building space; maintenance, ultra-cartridge plus CFC chip; calibration; network running cost; charging batteries; staff salary; staff training; utilities (water and power); and consumables (sample collection and biosafety requirements).
Costing approach
The bottom-up (ingredient costing) approach was used to determine the costs incurred when carrying out a single test if the same sample is subjected to either Xpert MTB/Rif test or Xpert Omni. Microcosting was conducted by exhaustively identifying all the inputs and their specific quantities required to perform a single test in each test method. The resources used associated with the Xpert MTB/Rif test were measured through observation of standard operating procedures as the laboratory technician performed the tests at the hub. Staff salary was allocated to the time required to analyse one sample for Xpert MTB/Rif and one sample of Xpert Omni. For both Xpert Omni and Xpert MTB/Rif, a staff was assumed to work for 260 days annually. It required 2 hours and 40 min to prepare, incubate, process and generate results for four samples in the Xpert MTB/Rif. The Xpert Omni processes one sample at a time, and it requires approximately 1 hour and 30 min to prepare, incubate, analyse and record results.
The useful life span of both the Xpert MTB/Rif and Xpert Omni was assumed to be 5 years,22 27 and 100% of their use was allocated to TB testing. Based on the capacity of each machine to deliver results four times within eight working hours, the Xpert MTB/Rif test would perform an average of 16 tests per day, while Xpert Omni runs an average of four tests per day for all suspected TB cases irrespective of age and sex. The cost of the supplemental power battery for Xpert Omni was assumed to be 10% of the cost of the power backup of Xpert MTB/Rif. The manufacturer recommends an iPhone 5 as an appropriate mobile device for Xpert Omni to relay results, and thus, its cost was considered in the analysis. Network service costs were obtained from the Xpert Omni factsheet 2018.28
The motorcycle was used to transport sputum, blood, viral load and early infant diagnosis samples in varying quantities per month. A proportion of the useful life of the motorcycle, sample carrier, expenditure on the resources (fuel, maintenance and communication) and hub rider’s salary was allocated to sputum samples transported. The cost per sample was then determined by dividing the cost incurred for each cost item by the total number of sputum samples transported. Information from the riders’ register as of 30 April 2018 showed that an average of 244 sputum sample areas were transported to the GeneXpert site per month. This represents approximately 30% of all the sample categories transported to the hub. Therefore, 30% of the useful life of the motorcycle, sample carrier, expenditure on the resources (fuel, maintenance and communication) and hub riders’ salary were allocated to transporting sputum samples.
We assumed that one technician required a 5-day training in using Xpert Omni or Xpert MTB/Rif per year and that Xpert cartridge procurement and transportation would equal 10% of the US$10 cartridge list price, based on consultation with experts from National Tuberculosis Reference Laboratory and The AIDS Support Organisation. The cost of shipping the Xpert MTB/Rif or Xpert Omni system was incorporated into the original concessional price. Annual maintenance would be based on annual warranty cost with a 5-year expected lifetime.23 We assumed building space occupied by Xpert MTB/Rif to be 5% of the total cost each year given the assumed expected lifetime of 30 years, and Xpert Omni was assumed to take 10% of the space occupied by Xpert MTB/Rif.
Valuation and quantification of inputs
All costs were estimated as of mid-2018 prices and converted to US dollars using published exchange rates. The local costs were converted using the average exchange rate of 3730 Uganda shillings to US$1 as of 15 June 2018. Because of the differential timing of costs, all future costs were not discounted at recommended inflation discount rate of 3% per year29 because the time horizon was 1 month, and the valuation of inputs was done at one point in time in June 2018. A unit cost per test was calculated by dividing the cost of the equipment by the total adjusted number of tests it would perform in its useful lifetime. For example, an Xpert MTB/Rif machine has capacity to run 16 tests a day, 4160 tests per year and 20 800 tests in 5 years of its life. With the total cost of the machine being US$17 000, the unit cost per test would be 17 000/20 800 giving US$0.817. A similar approach was used in determining the costs allocated per test for all other equipment used in Xpert TB testing.
Decision analysis model for cost-effectiveness analysis
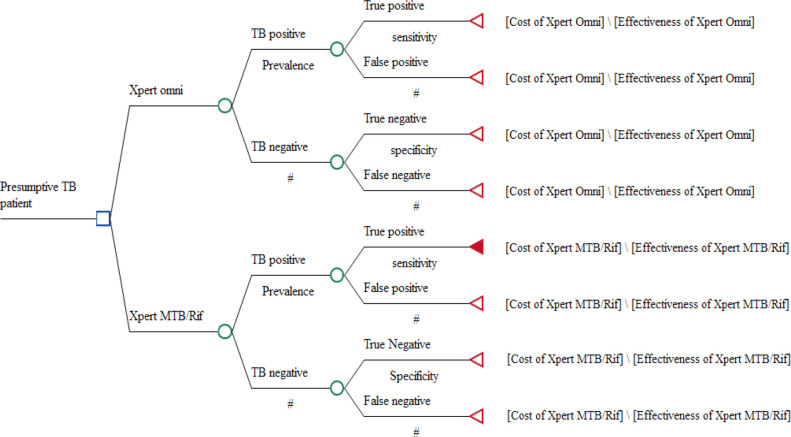
A decision tree analysis model (figure 1) was constructed using TreeAge V.2015 software (TreeAge Pro Inc, Williamston, Massachusetts, USA) to compare the diagnostic outcomes and costs of GeneXpert MTB/Rif and GeneXpert Omni. A positive test was either a true positive or a false negative based on the sensitivity of Xpert Omni or Xpert MTB/Rif. A negative test was either a true negative or a false positive based on the specificity of Xpert Omni or Xpert MTB/Rif.
Figure 1.
Decision analysis model for cost-effectiveness analysis.
Sensitivity analysis
One-way deterministic sensitivity analysis was performed in the TreeAge software to identify the most influential model parameters and to test the robustness of the model. Uncertainty was centred on the costs, sensitivity and specificities of the Xpert MTB/Rif and Xpert Omni. A parameter was considered sensitive if the possible changes altered the incremental cost-effectiveness ratio (ICER) sufficiently to switch preference to the alternative. Sensitivity analysis was majorly centred on the costs because the values were majorly obtained from expert opinion and fluctuating market prices. Costs for both tests were reduced by half and increased by half to ascertain their impact on cost-effectiveness. Analysis was also performed on sensitivity, specificity and prevalence because the values used in the model were obtained from previous studies that gave varying values. Analysis was performed from the model by adjusting the model parameters of Xpert MTB/Rif and Xpert Omni tests based on the minimum and maximum values from published literature.8 16 27 30–33 TB prevalence corresponding to the national estimates at the time of the study obtained from reports and systematic reviews were used in the analysis.1 3 34
The useful life of each Xpert machine varied between 5 and 10 years. The average number of tests performed per day by Xpert MTB/Rif varied between 8 and 24 tests per day. The tests done by Xpert Omni varied between two and eight tests per day. Different prices of the standard and ultra-cartridges for Xpert MTB/Rif and Xpert Omni, respectively, were varied by using costs obtained from market prices at the time of the study. The percentage allocated for staff time varied from 10% to 30% due to fluctuations in the number of patients per day. TB prevalence varied from 159/100 000 to 253/100 000 as reported by Uganda’s national population-based TB prevalence survey (2014–2016).35
Effectiveness outcome measure
The model’s primary effectiveness outcome measure was incremental cost per additional PTB test diagnosed when the Xpert MTB/Rif test or Xpert Omni test was used for TB diagnosis among presumptive cases. A positive test was either a true positive or a false negative and a negative test was either a true negative or a false positive based on the sensitivity and specificity of Xpert Omni or Xpert MTB/Rif. Estimates for sensitivities and specificities of the tests were obtained from pooled values from the systematic reviews and clinical trials that took mycobacterial culture as a reference standard.16 30 31 33 TB prevalence from the Uganda national TB survey of 2016 was considered for analysis, and model probabilities (table 1) were entered and analysed in TreeAge Pro version 2017 software (TreeAge Pro Inc).
Cost-effectiveness outcome measure
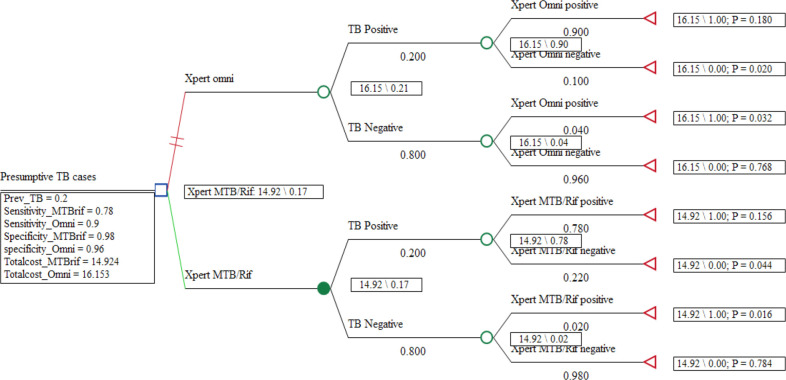
The cost-effective analysis outcome measure was incremental cost per additional Pulmonary Tuberculosis test diagnosed expressed as the ICER. The ICER was defined as the change in costs over the change in the effectiveness of moving from Xpert MTB/Rif to Xpert Omni expressed as USD per TB patient diagnosed. The cost per diagnostic outcome was used as a more proximal measure for exploring the key drivers of cost-effectiveness (figure 2).
Figure 2.
Rolled-back decision analysis tree comparing Xpert MTB/RIF and Xpert Omni.
Summary of assumptions
This study used the concessional prices of the Xpert MTB/Rif and Xpert Omni machines and cartridges that are provided for resource-poor settings. The study assumed that all the overhead costs of laboratory infrastructure, installation costs, sample collection requirements and biosafety requirements were equal for both tests. The useful life of the motorcycle and sample carrier was assumed to be 10 years as per the donor’s grant requirements to the implementing partner. A presumptive TB case was assumed to have had cough and fever for 2 weeks or more, excessive night sweats and a noticeable loss of weight or current cough for HIV-positive patients.3 A hypothetical population of 10 000 patients was used in the analysis to determine the effectiveness of the test methods under study.
Patient and public involvement
None.
Results
Cost per test for Xpert MTB/Rif and Xpert Omni
The total cost per test for Xpert MTB/Rif was US$14.933 (table 2). Cartridge and reagent kits contributed to 67% of Xpert MTB/Rif. Sample transport costs increased the cost per test of Xpert MTB/Rif by $1.28.
Table 2.
Cost estimates for Xpert MTB/Rif (US$)
| Inputs | Total cost | Cost per test | Source |
| Capital costs | |||
| Xpert MTB/Rif four module machine | 17 000 | 0.817 | Invoice |
| Power back-up | 2680.96 | 0.129 | Invoice |
| Printer | 100 | 0.005 | Invoice |
| Installation costs | 537.6 | 0.064 | Invoice |
| Building space allocation per test | 0.12 | Primary data | |
| Overhead costs | |||
| Standard cartridge and reagent | 10 | 10.0 | Invoice |
| Staff salary | 51 | 0.15 | Salary payslips |
| Aggregated transport costs per test | 1.28 | Primary data | |
| Calibration kit per 2000 tests | 450 | 0.225 | Invoice |
| Maintenance per year | 335 | 0.084 | Budget document |
| Training costs (5 days) per year | 284 | 0.85 | Budget document |
| Utilities (water, power) per month | 23 | 0.82 | Invoice |
| Aggregated consumables per test | 0.38 | Delivery notes | |
| Total cost per test | 14.924 |
Table 3 shows the total cost per test for Xpert Omni. Cartridge and reagent kits contributed to over 71.2% of Xpert Omni’s cost per test. The cost of the near field communication (NFC) chip increased the price of the Xpert Omni Ultra cartridge by $1.5.
Table 3.
Costs estimates for Xpert Omni (US$)
| Inputs | Total cost | Cost per test | Source |
| Capital costs | |||
| Xpert Omni machine and accessories | 5135 | 0.96 | 15 |
| Installation costs | 537.6 | 0.064 | Assumption* |
| Supplemental rechargeable battery | 268.1 | 0.052 | Assumption* |
| iPhone-5 | 649 | 0.031 | Local market costs |
| Building space allocation per test | 0.12 | Primary data | |
| Overhead costs | |||
| Ultra-cartridge plus CFC chip | 11.5 | 11.5 | (23) |
| calibration cartridge per 2000 tests | 450 | 0.225 | (23) |
| Network running cost estimate per year (Vodacom) | 550 | 0.55 | (33) |
| Cost of charging batteries | 0.27 | 0.067 | Expert opinion |
| Staff salary allocated for Omni test | 51 | 0.45 | Salary payslips |
| Maintenance per year | 335 | 0.084 | Assumption* |
| Training costs (5 days) per year | 284 | 0.85 | Assumption* |
| Utilities (water, power) per month | 23 | 0.82 | Assumption* |
| Consumables per test | 0.38 | Assumption* | |
| Total per test | 16.153 |
*Assumption shows the costs of Omni that are assumed to be equal to the costs of Xpert MTB/Rif.
Effectiveness and cost-effectiveness
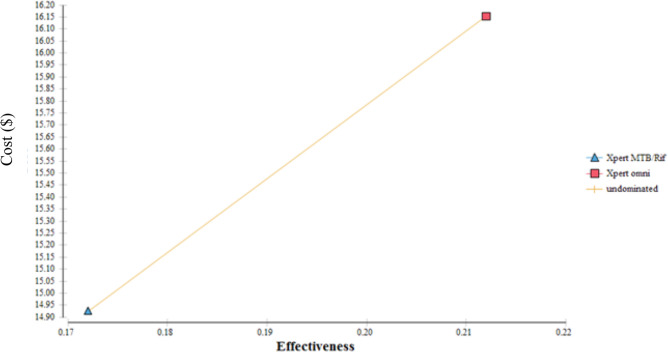
In the analysis involving a cohort of 10 000 presumptive TB cases with an average prevalence of 20% among HIV-positive patients, the test method using Xpert Omni would diagnose 2120 patients compared with 1720 patients diagnosed by Xpert MTB/Rif (table 4). The average cost-effectiveness of using Xpert MTB/Rif was US$86.77 per TB patient diagnosed compared with US$76.19 when Xpert Omni was used. The ICER for using Xpert Omni as a replacement for Xpert MTB/Rif was US$30.73 per additional case detected. There was no dominance noted in the cost-effectiveness analysis meaning no strategy was dominant over the other. From the cost-effectiveness plane shown in figure 3, it is observed that the GeneXpert Omni is more effective but with an increased cost compared with Xpert MTB/Rif although Xpert MTB/Rif was not dominated.
Table 4.
Cost-effectiveness table comparing Xpert MTB/Rif and Xpert Omni
| Strategy | Cost | Inc.cost | Eff | Inc.eff | Av. CER | ICER | Dominance |
| Xpert MTB/Rif | 149 240 | 1720 | 0 | 86.77 | Undominated | ||
| Xpert Omni | 161 530 | 12 290 | 2120 | 400 | 76.19 | 30.73 | Undominated |
Av.CER, average cost-effectiveness ratio; Eff, effectiveness; ICER, incremental cost-effectiveness ratio; Inc.cost, incremental cost; Inc.eff, incremental effectiveness.
Figure 3.
Cost-effectiveness plane.
Sensitivity analysis
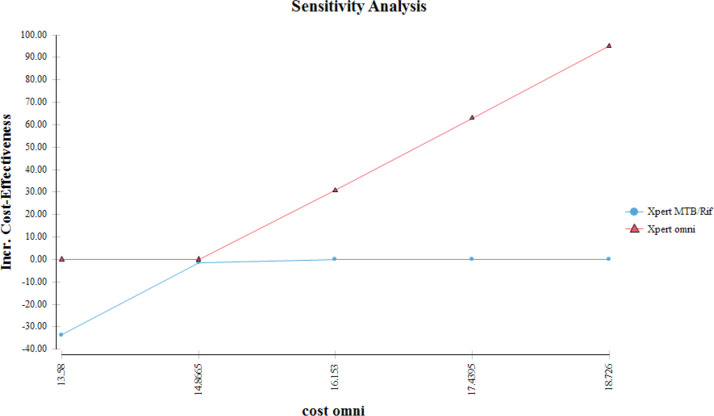
Sensitivity analysis was done on the input costs and probability variables in the model for both Xpert MTB/Rif and Xpert Omni (figure 4). This was done to determine which variables had an impact on the ICER. All variables were assigned too low and high ranges as shown in table 1. The model remained robust for plausible variations in the sensitivities, specificities and prevalence. The model was however sensitive to variations in the costs over time when adjusted for differential timing of the useful life of the GeneXpert machines (figure 4).
Figure 4.
Sensitivity analysis.
Discussion
Findings show the average cost-effectiveness of using Xpert MTB/Rif was US$86.77 per TB patient diagnosed compared with US$76.19 when Xpert Omni was used. The findings indicate that Xpert Omni would be a more effective strategy at a point of care. Therefore, the placement of Xpert Omni at POC could facilitate increased TB case detection. TB diagnosis in patients who harbour lesser quantities of the bacilli such as children, HIV positive cases and sputum smear-negative presumptive cases. This would in turn reduce early morbidity.36 Related studies that compared Xpert MTB/Rif to culture had found that Xpert was the least costly at reducing early mortality.37 38
The ICER for using Xpert Omni as a replacement for Xpert MTB/Rif was US$30.73 per additional case detected. This means that it shall require an additional US$30.73 for every additional TB case diagnosed by Xpert Omni. The Xpert Omni was however not dominant over the Xpert MTB/Rif. There are no cost-effectiveness studies published currently that have reported ICERs comparing Xpert Omni to Xpert MTB/Rif. However, studies that have compared the Xpert Ultra cartridge used in Omni and Xpert MTB/Rif cartridge have found the Ultra cartridge more effective in detecting PTB and rifampicin resistance. The Xpert Ultra cartridge was reported to be superior to the standard cartridge for TB case detection in participants with sputum smear-negative pulmonary TB. Xpert Ultra had superior sensitivity for TB case detection in HIV-infected persons.16 36
The study found that the estimated average total cost per test for Xpert MTB/Rif was US$14.924 and US$16.153 for Xpert Omni. This finding of the cost of Xpert MTB/Rif is slightly higher than that reported in a previous evaluation study in Uganda that found that the average cost of an Xpert MTB/Rif was US$12.41.24 However, other similar evaluations based on the observed mean monthly volume of 54 tests per site found the mean unit cost of Xpert to be US$21.22 In this study, however, the cartridge and reagent kit costs accounted for 67% of Xpert MTB/Rif and over 71% of Xpert Omni cost per test less than 84% reported in Walusimbi’s study for Xpert MTB/Rif. The evidence the cost of Xpert per test at POC in South Africa was higher than our estimates due to the increased price of the cartridge and reagent kit, which was set at $14.00. The cost per test of Xpert MTB/Rif was found to be US$26.54 for laboratory placement and US$38.91 for point-of-treatment placement.39
This study suggests that although the Xpert Omni test has cost implications, its use at POC would improve the diagnostic capacity, supplement the use of ZN microscopy for diagnosis, reduce the number of sputum samples collected, reduce the need for sputum sample transportation, reduce the median time to treatment initiation and ultimately reduce pretreatment loss to follow-up resulting from prolonged waiting for results after referral of samples. Implementation of Xpert Omni is likely to enable immediate identification of both non-drug and drug-resistant TB and improve the proportion of patients initiated on treatment. However, to maximise patient-level outcomes of the new diagnostic tool, it will require improvements in monitoring treatment through follow-up tests, interventions to reduce pretreatment loss to follow-up and time-to-treatment initiation.
This study had some limitations. First, the study included costs of Xpert MTB/Rif and Xpert Omni estimating diagnostic costs based on only the service providers’ perspective. It is therefore possible that we underestimated the true diagnostic costs faced in the implementation of the Xpert MTB/Rif and likely costs by Xpert Omni, especially by the society. Second, since the study did not measure the costs and outcomes related to treatment, survival and disability, cost-effectiveness was not measured in terms of cost per disability-adjusted life year averted, which are more robust measures of cost-effectiveness. The results maybe further weakened due to the fact probabilistic sensitivity analysis to quantify the amount of uncertainty and produce cost-effectiveness based on willingness to pay was not done. Third, the cost-effectiveness analysis is quite limited as it included only male Tuberculosis - HIV coinfected patients; thus, it is difficult to generalise this study to other populations with different prevalence rates.
Xpert Omni is an innovation with limited literature and robust supporting evidence for its adoption in the national TB testing algorithm. These Cost Effectiveness Analysis (CEA) results should only be interpreted and implemented with supporting evidence from robust studies such as the Xpert Omni performance (XPEL-TB) parallel cluster-randomised study in Uganda and another pragmatic cluster-randomised controlled trial of GeneXpert Omni combined with the Xpert MTB/Rif Ultra for detection of TB and rifampicin resistance in adults with presumptive pulmonary TB at primary-level diagnostic centres in Tanzania. Further research is therefore needed to evaluate the performance of the GeneXpert machine in the local setting. Since our analysis focused on a specific population with a prevalence of TB as at the time of data collection, these results are bound to change with changes in prevalence rates from other populations. Further research on the general population is needed to provide generalisable findings. However, these findings are indicative of Xpert Omni being a potential cost-effective point of care test compared with Xpert MTB/Rif.
Supplementary Material
Acknowledgments
Management and administration of Serere District Health Office, Serere Health Center IV and Pingire Health Center III who provided all the support for the research.
Footnotes
Contributors: DLE collected the data, performed the analysis, wrote the report and drafted the manuscript. DLE guaranteed and accepted full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish. RK and AI supported in data collection, analysis and report writing and manuscript drafting. JN and ADM extensively reviewed the report and manuscript. JK and EE supervised the entire research process, reviewed the report and manuscript and gave final approval for submission and publication.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data sharing not applicable as no datasets generated and/or analysed for this study. No data are available.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Ethical clearance for the study was issued by the higher degrees, research and ethics committee of Makerere University School of Public health. An ethical approval ID was not obtained since board does not issue IDs to master students’ approved research. Participants who gave their opinions signed a written informed consent before participating. Participants gave informed consent to participate in the study before taking part.
References
- 1.WHO . Global tuberculosis report 2021 [Internet], 2021. Available: https://www.who.int/publications/i/item/9789240037021 [Accessed 27 Jun 2022].
- 2.UPHIA . UPHIA_Final_Report_Revise_07.11.2019_Final_for-web.pdf [Internet], 2019. Available: https://phia.icap.columbia.edu/wp-content/uploads/2019/07/UPHIA_Final_Report_Revise_07.11.2019_Final_for-web.pdf [Accessed 30 Mar 2022].
- 3.MOH . The Uganda National Tuberculosis Prevalence Survey, 2014-2015 Survey Report [Internet]. Ministry of Health | Government of Uganda, 2016. Available: https://www.health.go.ug/cause/the-uganda-national-tuberculosis-prevalence-survey-2014-2015-survey-report/ [Accessed 22 Oct 2020].
- 4.Kakame KT, Namuhani N, Kazibwe A, et al. Missed opportunities in tuberculosis investigation and associated factors at public health facilities in Uganda. BMC Health Serv Res 2021;21:359. 10.1186/s12913-021-06368-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MOH . Annual Health Sector Performance Report Financial Year 2016/2017 | Ministry of Health Knowledge Management Portal [Internet], 2017. Available: http://library.health.go.ug/publications/annual-quarterly-performance-reports/annual-health-sector-performance-repor-t-financial [Accessed 26 Apr 2022].
- 6.WHO . Global tuberculosis control: surveillance, planning and financing. WHO/HTM/TB/2006.362. Geneva: WHO. Geneva, 2006. [Google Scholar]
- 7.WHO . Automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF assay for the diagnosis of pulmonary and extrapulmonary TB in adults and children. Policy update. [Internet], 2013. Available: http://who.int/tb/laboratory/xpert_policyupdate/en/ [PubMed]
- 8.Boehme CC, Nicol MP, Nabeta P, et al. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. Lancet 2011;377:1495–505. 10.1016/S0140-6736(11)60438-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ocheretina O, Byrt E, Mabou M-M, et al. False-Positive rifampin resistant results with Xpert MTB/RIF version 4 assay in clinical samples with a low bacterial load. Diagn Microbiol Infect Dis 2016;85:53–5. 10.1016/j.diagmicrobio.2016.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blakemore R, Story E, Helb D, et al. Evaluation of the analytical performance of the Xpert MTB/RIF assay. J Clin Microbiol 2010;48:2495–501. 10.1128/JCM.00128-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rufai SB, Kumar P, Singh A, et al. Comparison of Xpert MTB/RIF with line probe assay for detection of rifampin-monoresistant Mycobacterium tuberculosis. J Clin Microbiol 2014;52:1846–52. 10.1128/JCM.03005-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williamson DA, Basu I, Bower J, et al. An evaluation of the Xpert MTB/RIF assay and detection of false-positive rifampicin resistance in Mycobacterium tuberculosis. Diagn Microbiol Infect Dis 2012;74:207–9. 10.1016/j.diagmicrobio.2012.06.013 [DOI] [PubMed] [Google Scholar]
- 13.Cox HS, Mbhele S, Mohess N, et al. Impact of Xpert MTB/RIF for TB diagnosis in a primary care clinic with high TB and HIV prevalence in South Africa: a pragmatic randomised trial. PLoS Med 2014;11:e1001760. 10.1371/journal.pmed.1001760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trajman A, Durovni B, Saraceni V, et al. Impact on Patients’ Treatment Outcomes of XpertMTB/RIF Implementation for the Diagnosis of Tuberculosis: Follow-Up of a Stepped-Wedge Randomized Clinical Trial. PLoS One 2015;10:e0123252. 10.1371/journal.pone.0123252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cepheid. Cepheid . Genexpert Omni: the true point of care molecular diagnostic system: Cepheid Inc, 20152015. Available: http://www.cepheid.com/us/genexpert-omni
- 16.Dorman SE, Schumacher SG, Alland D, et al. Xpert MTB/RIF ultra for detection of Mycobacterium tuberculosis and rifampicin resistance: a prospective multicentre diagnostic accuracy study. Lancet Infect Dis 2018;18:76–84. 10.1016/S1473-3099(17)30691-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO . Meeting report of a technical expert consultation: non-inferiority analysis of Xpert MTB/RIF ultra compared to Xpert MTB/RIF, 2017. [Google Scholar]
- 18.Opio A, Muyonga M, Mulumba N. HIV infection in fishing communities of Lake Victoria Basin of Uganda--a cross-sectional sero-behavioral survey. PLoS One 2013;8:e70770. 10.1371/journal.pone.0070770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boehme CC, Nabeta P, Hillemann D, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med 2010;363:1005–15. 10.1056/NEJMoa0907847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Rie A, Page-Shipp L, Hanrahan CF, et al. Point-Of-Care Xpert® MTB/RIF for smear-negative tuberculosis suspects at a primary care clinic in South Africa. Int J Tuberc Lung Dis 2013;17:368–72. 10.5588/ijtld.12.0392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steingart KR, Schiller I, Horne DJ. Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. In: Cochrane Database of Systematic Reviews [Internet. Chichester, UK: John Wiley & Sons, Ltd, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsiang E, Little KM, Haguma P, et al. Higher cost of implementing Xpert(®) MTB/RIF in Ugandan peripheral settings: implications for cost-effectiveness. Int J Tuberc Lung Dis 2016;20:1212–8. 10.5588/ijtld.16.0200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.FIND . GeneXpert® Negotiated prices [Internet]. FIND, 2018. Available: https://www.finddx.org/pricing/genexpert/ [Accessed 22 Oct 2020].
- 24.Walusimbi S, Kwesiga B, Rodrigues R, et al. Cost-Effectiveness analysis of microscopic observation drug susceptibility test versus Xpert MTB/RIF test for diagnosis of pulmonary tuberculosis in HIV patients in Uganda. BMC Health Serv Res 2016;16:563. 10.1186/s12913-016-1804-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.NMS . National Medical Stores Uganda [Internet, 2018. Available: https://www.nms.go.ug/index.php/component/content/featured?id=featured&start=35 [Accessed 27 Apr 2022].
- 26.JMS . Instruments, Equipment & Spare parts [Internet], 2018. Available: https://www.jms.co.ug/index.php/e-shop/instruments-equipment-spare-parts [Accessed 27 Apr 2022].
- 27.Pantoja A, Fitzpatrick C, Vassall A, et al. Xpert MTB/RIF for diagnosis of tuberculosis and drug-resistant tuberculosis: a cost and affordability analysis. Eur Respir J 2013;42:708–20. 10.1183/09031936.00147912 [DOI] [PubMed] [Google Scholar]
- 28.Medecins Sans Frontieres . Xpert Omni Facctsheet; MSF concerns on suitability for context, 2018. [Google Scholar]
- 29.Edejer TTT. Making choices in health: who guide to cost-effectiveness analysis. Geneva: World Health Organization, 2003: 312. [Google Scholar]
- 30.Vassall A, van Kampen S, Sohn H, et al. Rapid diagnosis of tuberculosis with the Xpert MTB/RIF assay in high burden countries: a cost-effectiveness analysis. PLoS Med 2011;8:e1001120. 10.1371/journal.pmed.1001120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trajman A, Durovni B, Saraceni V, et al. Impact on patients' treatment outcomes of XpertMTB/RIF implementation for the diagnosis of tuberculosis: follow-up of a Stepped-Wedge randomized clinical trial. PLoS One 2015;10:e0123252. 10.1371/journal.pone.0123252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steingart KR, Schiller I, Horne DJ, et al. Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev 2014:CD009593. 10.1002/14651858.CD009593.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.WHO . Global tuberculosis report 2016 [Internet. World Health Organization, 2016: 142. https://apps.who.int/iris/handle/10665/250441 [Google Scholar]
- 34.Mitku AA, Dessie ZG, Muluneh EK, et al. Prevalence and associated factors of TB/HIV co-infection among HIV infected patients in Amhara region, Ethiopia. Afr Health Sci 2016;16:588–95. 10.4314/ahs.v16i2.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MOH . The Uganda National Tuberculosis Prevalence Survey, 2014-2015 Survey Report [Internet]. Ministry of Health | Government of Uganda, 2016. Available: https://www.health.go.ug/cause/the-uganda-national-tuberculosis-prevalence-survey-2014-2015-survey-report/ [Accessed 21 Oct 2020].
- 36.Chakravorty S, Simmons AM, Rowneki M, et al. The New Xpert MTB/RIF Ultra: Improving Detection of Mycobacterium tuberculosis and Resistance to Rifampin in an Assay Suitable for Point-of-Care Testing. mBio 2017;8:e00812–7. 10.1128/mBio.00812-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Auld AF, Fielding KL, Gupta-Wright A, et al. Xpert MTB/RIF - why the lack of morbidity and mortality impact in intervention trials? Trans R Soc Trop Med Hyg 2016;110:432–44. 10.1093/trstmh/trw056 [DOI] [PubMed] [Google Scholar]
- 38.Abimbola TO, Marston BJ, Date AA, et al. Cost-Effectiveness of tuberculosis diagnostic strategies to reduce early mortality among persons with advanced HIV infection initiating antiretroviral therapy. J Acquir Immune Defic Syndr 2012;60:e1–7. 10.1097/QAI.0b013e318246538f [DOI] [PubMed] [Google Scholar]
- 39.Schnippel K, Meyer-Rath G, Long L, et al. Scaling up Xpert MTB/RIF technology: the costs of laboratory- vs. clinic-based roll-out in South Africa. Trop Med Int Health 2012;17:1142–51. 10.1111/j.1365-3156.2012.03028.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mitku AA, Dessie ZG, Muluneh EK, et al. Prevalence and associated factors of TB/HIV co-infection among HIV infected patients in Amhara region, Ethiopia. Afr Health Sci 2016;16:588. 10.4314/ahs.v16i2.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing not applicable as no datasets generated and/or analysed for this study. No data are available.