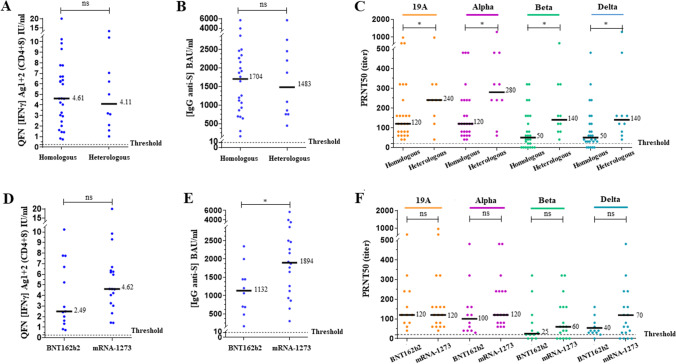
Fig. 5.
Variation in response according to vaccination scheme in non-infected individuals after 2 doses in the same age group. The median of each subgroup is represented by a black line with the specified value. Positive threshold of each test is represented by a dotted line. A, D Plasmas samples were assayed for QuantiFERON SARS-CoV-2 Starter Set® (QFN) Ag1 + 2. Data show the concentration of IFNγ (IU/ml), and are expressed as dot plots, one dot corresponding to one patient, with a positive threshold at 0.25 IU/ml. B, E Sera were assayed for S (RBD)-specific IgG. Data show the concentration (BAU/ml) of immunoglobulins, and are expressed as dot plots, one dot corresponding to one patient. Positive threshold is 8.5 BAU/ml (supplier’s threshold). C, F Sera were assayed for their capacity to neutralize the infection of Vero E6 cells by different SARS-CoV-2 strains, as indicated. Data show the PRNT50 titer and are expressed as dot plots, one dot corresponding to one patient. Positive threshold corresponds to the limit of detection at 20. A–C All results obtained 2–4 weeks after the second dose in non-infected patients aged under 55 years are divided into 2 groups of corresponding vaccination scheme, as indicated. Homologous scheme includes patients with identical prime and boost dose of BNT162b2 or mRNA-1273 vaccine. Heterologous scheme includes patients with a prime dose of AZD1222 followed by an mRNA boost. D–F All results obtained 2–4 weeks after the second dose in non-infected patients aged under 65 years are divided into 2 groups of corresponding mRNA vaccine type in the homologous scheme, as indicated. No statistical significance (ns, p > 0.05) and statistical significance are shown (Kruskal–Wallis’s test, *p < 0.05)