Abstract
Over the past few decades, immunotherapy has revolutionized the modern medical oncology field. Chimeric antigen receptor (CAR)-T cell therapy has a promising curative effect in the treatment of hematological malignancies. Anti-CD19 CAR-T cells are the most mature CAR-T cells recently studied and in recent years it has achieved a complete remission rate of approximately 90% in the treatment of B-cell acute lymphoblastic leukemia (B-ALL). Although CAR-T cell therapy has greatly alleviated the disease in patients with leukemia or lymphoma, some of them still relapse after treatment. Therefore, in this article, we discuss the factors that may contribute to disease relapse following CAR-T cell therapy and summarize potential strategies to overcome these obstacles, thus providing the possibility of improving standard treatment regimens.
Keywords: CAR-t, CD19, B-ALL, hematological malignancies, relapse, antigen escape
Introduction
Chimeric antigen receptor (CAR)-T cells are produced by gene editing T cells from the patients or donors to get the CAR-targeting antigens to kill corresponding cells. These cells are then transfused into the patient after they are expanded to a certain degree in vitro. The CAR is typically composed of 3 functional domains: the extracellular domain, transmembrane domain, and intracellular domain. The extracellular domain includes the single-chain variable fragment (scFv) from the monoclonal antibody that binds to the target and a hinge region that acts as a link. The transmembrane domain connects the extracellular domain of the CAR with the intracellular signal transduction domain and anchors the receptor to the T-cell membrane. The intracellular domain consists of the costimulatory domain and a signal transduction domain. The costimulatory domain is normally derived from the CD28 receptor family or the tumor necrosis factor (TNF) receptor family, such as CD28 or 4-1BB(CD137), which are components of CAR-T cells approved by the FDA for clinical use. The signal transduction domain is usually the T-cell receptor (TCR)/CD3ζ chain. 1
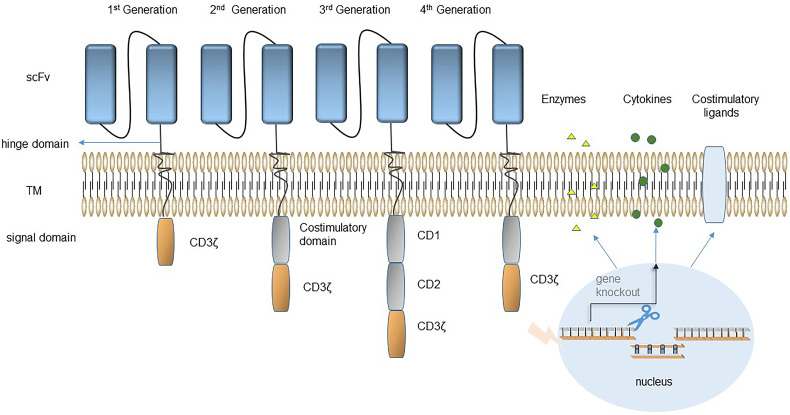
The CAR-T cells can be divided into 4 generations depending on the intracellular domains (Figure 1). The CD3-ζ chain was treated as the unique signal transduction domain by first-generation CAR-T cells, which showed limited expansion and durability and had poor antitumor effects in clinical trials. 2 Second-generation CAR-T cells have an additional costimulatory molecule in the intracellular domain, usually CD28 or 4-1BB, and these cells are the most widely used. As of 2022, the 6 CAR-T products approved for marketing by the FDA are all second-generation CAR-T cells, which have shown outstanding clinical therapeutic effects (Table 1). The intracellular structure of third-generation CARs has 2 or more stimulating molecules. Although it has shown great efficiency in some studies, the comprehensive analysis of the third-generation CAR-T cells are still limited, and whether to improve their efficiency is still controversial.3–5 Fourth-generation CAR-T cells are also known as “TRUCK-T cells”. TRUCK-T cells improve the antitumor ability and safety of CAR-T cells by modifying the CAR structure using secreted molecules and cell ligands. 6 Cytokine-armored CAR-T cells are the most common type of TRUCK-T cells. Sequences encoding cytokines (eg, IL-2, IL-7, IL15) were added to CAR sequences and introduced into T cells. The cytokines secreted by activated CAR-T cells participate in antitumor activities. They not only promote CAR-T cells differentiation, but also play a role in resisting tumor microenvironment inhibition.7,8 Agonist-armored CAR-T cells are also entering the scope of research. CAR-T cells are modified to express costimulatory ligands (CD40L or 4-1BBL). Recent clinical trials of anti-CD19 4-1BBL armored CAR-T cells for non-Hodgkin's lymphoma (NHL) or chronic lymphocytic leukemia (CLL) have been carried out, and achieved a complete response (CR) rate of 57% without serious adverse reactions, indicating that anti-CD19 4-1BBL armored CAR-T cells are effective and safe. 9
Figure 1.
Chimeric antigen receptors (CARs) can be divided into 4 generations based on their structure. The first generation of CAR structures contain only antigen-recognition signals. The second- and third-generation CARs have 1 and 2 co-stimulatory molecules added respectively in the signal transduction region to improve T-cell proliferation activity, and cytotoxicity, and prolong T-cell survival time. The fourth-generation CAR inserts additional molecular elements into the CAR to express functional transgenic proteins, such as secreted cytokines, suicide genes, and regulatory switches, to improve the effectiveness and safety of CAR-T cells.
Table 1.
Six CAR-T Products Approved by the FDA
| Trade name | Abbreviation | Approval time | Target | Viral vector | Costimulatory domain | Indication | Effect | References |
|---|---|---|---|---|---|---|---|---|
| Kymriah | Tiso-cel | 2017.08 | CD19 | Lentiviral | 4-1BB | Adult R/R DLBCL R/R B-cell precursor acute lymphoblastic leukemia (ALL) |
ORR:52% CRR:40% (n = 93) |
10 |
| Yescarta | Axi-cel | 2017.10 | CD19 | Retroviral | CD28 | R/R LBCL or FL | ORR:83% CRR:58% (n = 101) |
11 |
| Tecartus | Bre-cel | 2020.07 | CD19 | Retroviral | CD28 | Adult R/R MCL Adult R/R B-cell precursor acute lymphoblastic leukemia (ALL) |
ORR:93% CRR:67% (n = 60) |
12 |
| Breyanzi | Liso-cel | 2021.02 | CD19 | Lentiviral | 4-1BB | R/R DLBCL | ORR:73% CRR:53% (n = 256) |
13 |
| Abecma | Ide-cel | 2021.03 | BCMA | Lentiviral | CD28 | Adult R/R MM | ORR:73% CRR:33% (n = 128) |
14 |
| Carvykti | Cilta-cel | 2022.03 | BCMA | Lentiviral | 4-1BB | Adult R/R MM | ORR:97% CRR:67% (n = 97) |
15 |
Abbreviations: R/R, relapsed/refractory; DLBCL, diffuse large B-cell lymphoma; LBCL, large B-cell lymphoma; FL, follicular lymphoma; MCL, mantle cell lymphoma; MM, multiple myeloma; ORR, objective response rate; CRR, complete response rate.
In 2012, Emily Whitehead, a child patient with B-cell acute lymphoblastic leukemia (B-ALL), joined a phase I clinical project (CTL019) of Novartis after 2 relapses. After CTL019 cells infusion, grade 3∼4 adverse events occurred, mainly cytokine release syndrome (CRS), occurred. She has achieved morphological remission of leukemia approximately 1 month after infusion, and sustained remission for nearly ten years, becoming the first patient with B-ALL who benefited from CAR-T cell therapy. 16 Initial tests of anti-CD19 CAR-T cells for the treatment of refractory and/or relapsed (R/R) CD19-expressing B-cell malignancies have also achieved unprecedented success.17–19 Thereafter, a large number of clinical studies showed a complete remission rate of 70%-90% in pediatric and adult B-ALL patients treated with anti-CD19 CAR-T cell therapy, and the CR rate for patients with B-cell lymphoma reached approximately 50%.20,21 Although anti-CD19 CAR-T cells are currently the most mature and most effective CAR-T cells, analysis of follow-up data has shown that 30% to 50% of the treated patients relapsed after receiving anti-CD19 CAR-T treatment in CR, and most of them relapsed within 1 year. 21 According to the presence of the CD19 antigen, relapse can be divided into 2 modes: CD19-positive and CD19-negative relapse. The main reason for CD19-positive relapse is the poor persistence of CAR-T cells, and in such cases, the CD19 antigen is still preserved on the surface of tumor cells. 22 In the case of CD19-negative relapse, the CD19 antigen no longer exists, and the tumor evades the recognition and clearance of CAR-T cells, which is called antigen escape.17,23
Antigen-Positive Relapse
Mechanisms of Antigen-Positive Relapse
Generally speaking, early ALL relapse occurs within a few months after remission induction. 24 It is usually associated with the function of T cells, CAR-T cell acquisition, limited persistence of CAR-T cells, and transient B-cell regeneration, which indicate the loss of immune surveillance mediated by CAR-T cells. 17
Function of T Cells and Preparation of CAR-T Cells
Most patients have had intensive therapy before receiving CAR-T cells, reducing the number and impairing the function of patients’ own CD3+ T cells, which serve as the source for CAR-T cell preparation. 24 Das et al isolated peripheral blood T cells from 157 pediatric tumor patients and stimulated the expansion of these T cells with CD3/CD28 magnetic beads. The potential for CAR-T cells is very poor in all types of tumors except in prechemotherapy settings and retinoblastoma. Chemotherapeutic agents such as cyclophosphamide and doxorubicin can also lead to mitochondrial dysfunction in patient T cells and preferentially reduce naïve T lymphocytes with higher amplification capacity. 25 Various metabolites, inhibitory cytokines, immunosuppressive cells, and immunosuppressive molecules in tumor-suppressive microenvironments also affect the antitumor activity of T cells.26,27 For example, upregulation of inhibitory molecules (PD-1, TIM-3, CTLA-4, and LAG-3) on CAR-T cells results in functional inhibition and failure of T cells.10,28 Interestingly, activation of PD-1 preferentially inhibits CD28 CAR-T cells. 29
Preparing CAR-T cells requires a complex process, including apheresis, sorting, and transfection. Deficiency of any of these steps may lead to the immaturity of CAR-T cells and affect proliferation and function.30,31 Jin et al 32 first reported the effect of infection temperature differences on the phenotype and function of CAR-T cells and suggested lentivirus infection at 32 degrees to prepare CAR-T cells. In addition, with the gradual industrialization of CAR-T cells’ manufacturing, it is necessary to freeze peripheral blood cell materials and CAR-T cells for storage. How cell viability and phenotype changed during freezing or thawing has been studied.33,34 In a clinical experiment that compared the utility of fresh and frozen/thawed bispecific anti-CD19/CD20 CAR-T cells for treating R/R NHL, Shah et al 35 found that the average viability of cryopreserved CAR-T cells at the time of infusion was significantly lower than that of fresh CAR-T cells. Moreover, in patients receiving fresh products, the expansion, activity, and persistence of CAR-T cells showed an increasing trend.
Limited Persistence of CAR-T Cells
The phenotype of CAR-T cells before and after preparation will affect their expansion and persistence to some extent. Children's CAR-T cells tend to have a stronger proliferation ability than adults’ because they contain a higher proportion of naïve T cells which have an immature phenotype to be the best source for producing CAR-T cells.36,37 There is evidence that CAR-T cells prepared in vitro contain more cells with an early phenotype (naive T cells and central memory T cells), which have superior proliferation capacity, persistence, and antitumor effects in vivo. 38 Alternatively, the ratio of CD4+ and CD8+ T cells in CAR-T cells may also influence their final function. 39 Turtle et al 40 manufactured CAR-T cell products with a CD4+ to CD8+ ratio of 1:1 in their experiments. After infusing those cells into R/R B-ALL patients, 27 of 29 patients (93%) achieved bone marrow remission.
Previous clinical data indicated that the CAR-T cells containing the costimulatory molecule CD28 lasted shorter than CAR-T cells containing 4-1BB.21,41 This discrepancy may result from different metabolic characteristics between the 2.42,43 CD28 CAR-T cells primarily utilize glycolysis to meet the increased metabolic requirements of effector T cells. 44 4-1BB CAR-T cells, however, promote the growth of CD8+ central memory T cells, which have significantly enhanced respiratory capacity, increased fatty acid oxidation, and enhanced mitochondrial biogenesis. 45 Moreover, CD28 generally leads to more rapid tumor clearance with a more obvious decrease in memory T cells compared with 4-1BB. Salter et al 46 found that the same protein phosphorylation events occurred in both CD28 CAR-T and 4-1BB CAR-T cells. However, the signaling dynamics and intensity were higher upon CD28 CAR activation, whereas 4-1BB CAR-T cells showed slower expansion and lower signaling intensity. Lymphocyte-specific tyrosine kinase (Lck) is a kind of protein tyrosine kinase involved in the development and activation of T cells. The level of Lck in immunoprecipitates from unstimulated CD28 CAR-T cells was higher than that in immunoprecipitates from 4-1BB CAR-T cells. The enhanced signal strength of CD28 CAR-T cells is in part related to the constitutive binding of Lck to the CD28 domain. Researchers also found that 4-1BB CAR-T cells preferentially expressed T-cell memory-related genes after stimulation. Subsequently, Sun et al 47 found that Lck was recruited into the CD28 synapse of CARs via coreceptors, resulting in antigen-independent CAR-CD3ζ phosphorylation and increased activation of antigen-dependent T cells. In contrast, the synapses formed by 4-1BB CARs recruited the THEMIS-SHP1 phosphatase complex, which attenuated the phosphorylation of CAR-CD3ζ.
Furthermore, 4-1BB CAR-T cells have better persistence than CD28 CAR-T cells, possibly because CAR-T cells containing 4-1BB express higher levels of antiapoptotic proteins such as Bcl-2 and Bcl-XL and prevent T cells failure caused by the absence of ligand-dependent tetanic signals.48,49 Currently, the CAR-T cell scFv fragments used clinically are mostly derived from mice, whose high immunogenicity may lead to the rapid failure of CAR-T cells in humans.50,51 Other scholars have also found that low target antigen expression may damage anti-CD22 CAR-T cell function in vitro and in vivo and impair the persistence of CAR-T cells in vivo. 52
Prevention and Treatment Strategies for Antigen-Positive Relapse
The reinfusion of CAR-T cells is a possible clinical option for patients with disease relapse. Scholars from the University of Washington School of Medicine in the United States suggested that it is relatively safe and reliable to receive CAR-T cells again after initial CAR-T cell treatment. Long-lasting clinical remission can be achieved, especially in patients who receive an increased infusion dose and Cy-Flu pretreatment during CAR-T cell retreatment. 53
As mentioned earlier, patients generally received intensive treatment before CAR-T cell infusion. These treatments may affect the function and phenotype of CAR-T cells to some extent. Therefore, to obtain CAR-T cells of sufficient quantity and quality, we suggest that apheresis be employed whenever possible in patients with early-onset disease and those who have received lower doses of chemotherapy. CD4+ and CD8+ T cells are both effective cellular components of CAR-T cells, and their proportions vary greatly among different patients. Some scholars have suggested that the proportion affect the antitumor effect of CAR-T cells. In some experiments, CD4+ and CD8+ CAR-T cells have been delivered to patients at a determined ratio. 19 Lisocabtagene maraleucel (Liso-cel) is an autologous CAR-T cell therapy targeting CD19 developed by Juno. This therapy is unique compared to other CAR-T therapies already available because the proportion of CD8+ and CD4+ T cells are controlled (at a 1:1 ratio). Liso-cel is the fourth CAR-T cell product approved by the FDA. Its approval was based on the efficacy and safety demonstrated in the TRANSCEND NHL 001 clinical trial. The experimental data indicated that among 256 patients included in the efficacy assessment, 186 (73%) achieved objective remission, and 136 (53%) achieved CR. 54
How to optimize and transform of the CAR structure to prevent positive relapse is a popular research topic. In addition to 4-1BB and CD28, other costimulatory molecules and multiple costimulatory molecules are also being used in the construction of CARs to improve the durability of CAR-T cells. 55 The use of scFv of mouse-origin results in T-cell-mediated host immune system rejection of allogenic sequences after the infusion of CAR-T cells. 56 This problem can be resolved by replacing mouse scFv with human scFv.57–59
Joint application of immunomodulators such as immune checkpoint inhibitors (ICIs) can partially optimize the persistence and efficacy of CAR-T cells. 60 Preclinical and clinical data have shown that PD-1 expression increases during CAR-T cells expansion and there are also reports that PD-1 antibodies can reduce CAR-T cell failure.61,62 In addition to the combination of small-molecule inhibitors, researchers have also designed a strategy employing dominant-negative PD-1 armored anti-CD19 CAR-T cells, whose clinical trials have been conducted. This therapy produced an objective response rate (ORR) of 77.8% and a CR rate of 55.6% in 9 patients with R/R B-cell lymphoma. In patients with persistent CR, CAR-T cells were amplified after infusion and could continue to be detected after 12 months. 63 However, some studies have shown that PD-1 may play an important role in maintaining normal proliferation and differentiation of T cells. PD-1 silencing may inhibit the proliferative activity of T cells, thereby impairing the cytotoxicity of T cells. 64 This result indicated that PD-1 may not be absolutely harmful and may be essential for maintaining normal proliferative activity and antitumor function. This result improves the understanding of the function of PD-1 academia and has guiding significance for the clinical application of PD-1 inhibitors.
Antigen-Negative Relapse
Mechanism of Antigen-Negative Relapse
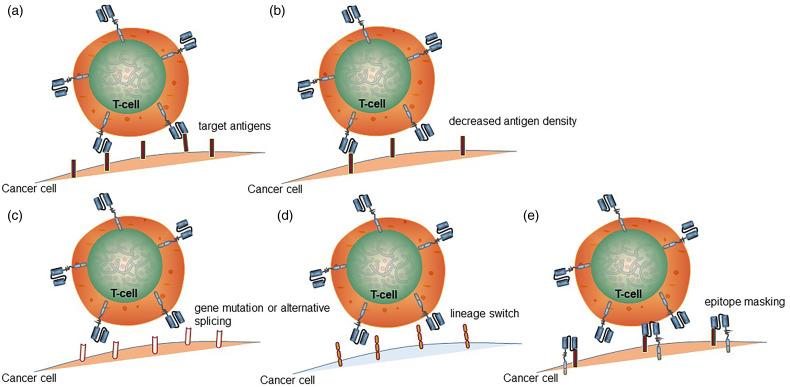
Due to the ongoing immunotherapy effects of CAR-T cells, tumor cells have developed mechanisms to evade CAR-T cells recognition and elimination, such as reduction in antigen density, mutation of antigen-encoding genes, alternative splicing, lineage switching, and epitope masking (Figure 2).
Figure 2.
Mechanism of antigen escape after chimeric antigen receptor (CAR)-T cell therapy. (a) CAR-T cells recognize and bind tumor-specific antigens to exert antitumor activity. (b) Reduced antigen density leads to antigen escape. (c) CAR mutation or alternative splicing, CAR-T cells cannot bind antigen. (d) The tumor surface antigen undergoes a lineage switch, and CD19 cannot bind to CAR-T cells. (e) For some reason, CAR metastasizes to the surface of tumor cells and binds to CD19 to mask its epitope, and CAR-T cells cannot attack the tumor.
Reduction in Antigen Density
Relapse in some patients does not necessarily manifest as a complete loss of antigen. The effective function of CAR-T cells requires antigen expression with a certain minimum threshold, and there are individual differences in this threshold. The downregulation of CD19 expression, that is, the reduction in CD19 antigen density, is sufficient to prevent CAR-T cell recognition and killing.65,66 Trogocytosis is a newly discovered mechanism related to the reduction in CD19 antigen density. CAR-T cells transfer the target antigens from tumor cells to the surface of CAR-T cells via trogocytosis, indicating that the loss of CD19 antigen expression is reversible. This process causes CAR-T cell “cannibalism,” which leads to increased T-cell exhaustion and decreased activity. 67 A similar mechanism was found in studies of anti-CD22 CAR-T cell therapy. 68 Reduced CD22 expression or antigen density on the surface of tumor cells is sufficient to prevent recognition and elimination by CAR-T cells. In this case, although CD22 expression was still positive, the patient also relapsed.
Gene Mutation and Alternative Splicing
Another mechanism is that tumor cells cannot be recognized and bound by CAR-T cells due to gene mutation or alternative splicing, even though CD19 still exists. Orlando et al 23 reported that 12 of the 17 patients with recurrent B-cell lymphoma did not have CD19 expression. Exon sequencing of these patients revealed that at least 1 frameshift mutation occurred per patient. These mutations result in the lack of transmembrane proteins on the cell surface and eventually the loss of function. The researchers also noted that the patients with CD19 antigen deletion mutations quickly relapsed. Such mutations are difficult to detect before relapse, and even 1 month before relapse.
Alternative splicing is also an important mechanism leading to antigen escape. Alternative splicing is a posttranscriptional mechanism that leads to the generation of unique mRNA transcripts encoding protein isoforms with different structures and functions, which enriches the diversity of proteins and plays an important role in human growth. 69 Alternative splicing causes the skipping of exon 2 of CD19 to form a mutant truncated protein. Thus, CAR-T cells targeting CD19 cannot identify the target antigen accurately, resulting in targeting errors. 70 In addition, such truncated proteins have been found not only in patients who have relapsed after CAR-T cell treatment but also in patients who have recently been diagnosed with leukemia. 71
Lineage Switching
Leukemia lineage switching is another mechanism that can lead to antigen escape. Lineage switching usually occurs in B-ALL or mixed-phenotype acute leukemia carrying mixed-lineage leukemia gene rearrangement on chromosome 11q23.72,73 Some preclinical and clinical studies have found a shift to a myeloid phenotype following anti-CD19 CAR-T cell treatment of B-cell hematological tumors.74,75 Turtle et al first reported an R/R CLL patient with lineage switching who relapsed and developed plasma cell lymphoma without CD19 expression after anti-CD19 CAR-T treatment. 76 Gardner et al 72 also reported that 2 of 7 B-ALL patients developed acute myeloid leukemia relevant to their B-ALL clone within 1 month after undergoing CD19 CAR-T cell transfusion.
Epitope Masking
Epitope masking is a rare antigen escape mechanism. For some reason, the CD19 antigen is recognized by CARs expressed on other cells, so the CAR-T cells cannot recognize and attack tumor cells. Ruella et al 77 identified a patient with antigen-negative relapse after CD19 CAR-T cell therapy. Flow cytometry did not detect CD19 antigen expression in bone marrow specimens but subsequently detected CD19 gene transcription products. Therefore, the researchers hypothesized that the undetectable expression of the CD19 antigen was due to epitope masking caused by CAR binding to the antigen. Subsequently, other experiments confirmed that the CAR gene had accidentally been introduced into individual leukemic B cells during the CAR-T cell preparation process; its product was thus expressed on the surface of the B cells, where it bound to CD19, preventing recognition by CAR-T cells. Monoclonal leukemia cells exhibited increased proliferation, leading to CD19-negative leukemia clones that were resistant to CAR-T cell therapy, thus CAR-T cells could not recognize CD19 and attack leukemia cells.
Survival Advantage of CD19-negative Subclones
It seems that antigen escape is not the only escape mechanism caused by treatment; the intrinsic heterogeneity of the initial leukemia clone is also obviously related to antigen-negative relapse. 78 There is evidence that a minority of B-ALL patients already have genetic variant subclones with negative or low CD19 expression at the time of initial diagnosis. 71 In these patients, after CAR-T cells eliminated cells with threshold CD19 antigen density (CD19-positive clones), CD19-negative clones still existed and gained a survival advantage. Ruella et al. 77 also found that there were rare CD19-CD123+ leukemia cells in the specimens of B-ALL patients. When injected into the immunodeficient mice, these cells can result in the re-establishment of the original B-ALL phenotype. 79
Prevention and Treatment Strategies for Antigen-Negative Relapse
Looking for New Targets
To overcome CD19 antigen escape, researchers are actively looking for other pan-B-cell antigens. CD22 is expressed in many patients with CD19-negative relapse, and anti-CD22 CAR-T cell therapy is also a frequently used strategy to overcome CD19-negative relapse.80,81 The preliminary results of clinical trials of anti-CD22 CAR-T cell treatment in patients with CD19-negative relapsed B-ALL were satisfactory. 73% (11/15) of all patients were minimal residual disease (MRD)-negative and achieved disease remission. 68 In a recent study, researchers from China identified 7 patients with R/R diffuse large B-cell lymphoma (DLBCL) and 6 patients with R/R B-ALL who received anti-CD19 CAR-T cell therapy, developed CD19-negative relapse and received anti-CD22 CAR-T cell salvage treatment. As a result, 6 of the DLBCL patients achieved CR, and 2 B-ALL patients achieved CR/complete remission with incomplete hematologic recovery. 82 In addition, preclinical studies of other potential target antigens, such as CD20, 83 thymic stromal lymphopoietin receptor (TSLPR), 84 and B-cell activating factor receptor 85 are also actively being carried out. However, due to the strong heterogeneity of tumors, targeting a single target to avoid antigen escape may still be insufficient to reduce relapse.
CAR-T Cells Targeting Multiple Antigens
The latest strategies for overcoming antigen escape focus on the generation of multitargeted CAR-T cells. Dual-target CAR-T cells can express 2 different CAR molecules and can target and kill tumor cells expressing either of the 2 target antigens, which provides coverage to multiple antigens 86 and reduces relapses caused by single-antigen escape to a certain extent.
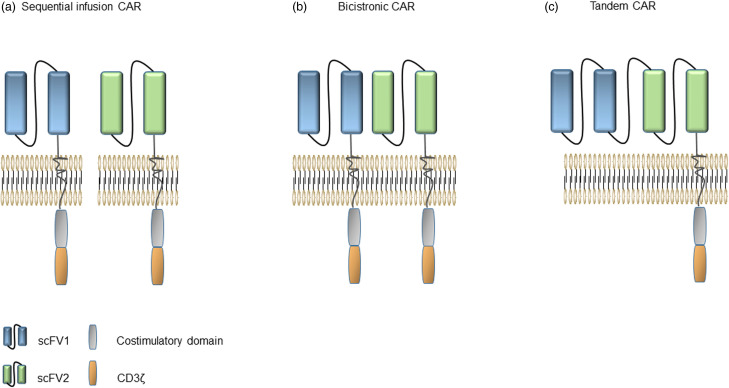
There are 3 main strategies employing dual targets of CAR-T cells 87 : sequential infusion with single-target CAR-T cells, co-expression of 2 different CARs in a T cell (bicistronic CAR), or construction of a single CAR with 2 separate scFvs in tandem (tandem CAR) (Figure 3). CD19 and CD20/CD22/CD123 are the most commonly used dual-target CAR-T cell targeting combinations (Table 2). Clinical studies have shown that the treatment of pediatric R/R B-ALL by orderly infusing CD19 and CD22 CAR-T cells can prevent antigen escape and prolong the duration of remission. 88 In a phase I clinical trial of CD19/CD22 dual-target CAR-T cells to treat B-ALL in China, all 6 patients obtained MRD-negative CR, and none of them developed central toxicity. 89 Coincidentally, in another clinical trial of patients with R/R invasive B-cell lymphoma treated with CD19/CD22 dual-target CAR-T cells, of the 16 eligible patients, 14 (87.5%) achieved an objective response, and 10 (62.5%) got achieved a CR. About the 2-year overall survival (OS) and PFS, patients with CR were significantly higher than patients without CR. 90 These results are sufficient to illustrate that CAR-T cells targeting CD19 and CD22 are effective and safe.
Figure 3.
Schematic drawings of 3 dual-target CAR-T structures. (a) Sequential infusion of CAR-T cells targeting 2 different antigens. (b) CARs of bicistronic CAR-T cells are expressed independently. (c) Tandem CAR has 2 independent scFv fragments, but shares the same co-stimulatory molecules and signaling domains.
Table 2.
Partial Published Clinical Trial of Dual-Target CAR-T for B-cell Malignancies.
| NCT number | Target | Pattern | Vector | Indication |
|---|---|---|---|---|
| NCT03097770 | CD19/CD20 | Tandem | Lentiviral | B-cell lymphoma |
| NCT03207178 | CD19/CD20 | Bicistronic | Lentiviral | DLBCL |
| NCT03185494 | CD19/CD22 | Tandem | Lentiviral | B-ALL |
| NCT03233854 | CD19/CD22 | Tandem | Lentiviral | B-cell malignancies |
| NCT03289455 | CD19/CD22 | Bicistronic | Retroviral | B-ALL |
| NCT03407859 | CD19/CD20/CD22/CD10 | Sequential infusion | Lentiviral | B-ALL |
| NCT03870945 | CD19/CD20 | Tandem | Lentiviral | B-NHL |
| NCT04792489 | CD19/CD20 | Tandem | Lentiviral | DLBCL |
Abbreviations: DLBCL, diffuse large B-cell lymphoma; B-ALL, acute B lymphoblastic leukemia; B-NHL, B-cell non-Hodgkin’s lymphoma; DLBCL, diffuse large B-cell lymphoma.
CD123 is a target antigen widely expressed on various hematological malignancy cells. 91 Surprisingly, it also exists on leukemia stem cells (LSCs). 92 LSCs are primitive cells with the characteristics of hematopoietic stem cells, which are resistant to chemotherapy, associated with poor prognosis and related disease relapse. 93 In a study by Ruella et al, 79 the expression of CD123 was detected in patients with CD19-negative relapse after anti-CD19 CAR-T cell therapy. Anti-CD123 CAR-T cells’ administration successfully induced CR in the CD19-negative relapse B-ALL mouse model, and the OS was significantly prolonged. The researchers also compared the efficacy of infusing CD19/CD123 dual-target CAR-T cells with sequential using anti-CD19 CAR-T cells with equal quantities and anti-CD123 CAR-T cells. The conclusion is that the dual-target CAR-T cells worked better. Qin et al 94 first introduced a D domain into the CAR structure and then constructed a CD19/CD123 tandem CAR with dd-cg06, which had a strong antitumor effect.
However, additional questions remain to be answered. Will tumor cells adapt to escape from simultaneous targeting of 2 target antigens due to the stress of a continuous antitumor response? If so, it may not be enough to target 2 antigens, and better antigen combinations need to be studied to prevent antigen loss or escape.
Combination With Other Drugs
Immune checkpoint molecules are expressed on immune cells and can regulate the degree of immune activation. Under normal circumstances, these checkpoints inhibit the function of T cells to prevent them from harming normal cells. When tumors develop, these checkpoints are used by tumor and nontumor cells in the microenvironment to induce immune escape. 60 PD-1 and PD-L1/L2 play an important role in the immune escape, tumor progression, and survival of various malignancies.95,96 ICIs (such as anti-PD-1/PD-L1/CTLA-4 monoclonal antibodies) can relieve the immunosuppressive effects of tumors and myeloid-derived suppressor cells to T cells by interacting with immune checkpoints. Mutations of the CD19 gene may lead to the emergence of novel tumor antigens. Although these new antigens cannot bind to anti-CD19 CAR-T cells, they can be amplified by ICIs to achieve a wider range of target antigen-related antitumor responses. The ZUMA-6 clinical trial is testing Yescarta® (Axi-cel) combined with the PD-1 inhibitor atezolizumab in treating refractory DLBCL. Despite the positive results obtained by some previous clinical trials, the CAR-T cell levels and efficacy of Axi-cel combined with Atezo were found to be similar to those of Axi-cel alone. 97 Therefore, it is unclear whether the combined application of PD-1 inhibitors can enhance the therapeutic effect of CAR-T cells and reduce antigen escape.
Aneta Ledererova and others established the first relapsed CLL mouse model after anti-CD19 CAR-T cell treatment. Up to 70% of treated mice eventually develop CD19-negative relapse a few weeks after the initial positive reaction. Researchers have found that the loss of CD19 is caused by DNA promoter methylation. It is important is that the loss of the expression is reversible by treatment with a demethylating agent. 98 These results may indicate that the use of demethylating drugs in combination with CAR-T cell therapy may prevent CD19-negative relapse, increasing the response rates as the result.
Low-dose Radiation
A higher dose of radiation can directly cause tumor cell death. It can increase the exposure of tumor antigens, enhance antigen presentation, enhance the immunogenicity of tumor cells with low antigen expression, and induce the production and recruitment of antitumor immune effector cells. 99 In contrast to existing endogenous immune cells, CAR-T cells specifically recognize tumor antigens in a way that is not limited by the major histocompatibility complex and is independent of antigen processing and presentation. 100 Therefore, the effect of higher-dose irradiation on CAR-T cells was not obvious.
TNF-related apoptosis-inducing ligand (TRAIL), a TNF superfamily member, is expressed in activated T cells. It can induce apoptosis by binding with its related death receptors DR4/DR5. 101 Low-dose radiation can make native antigen-negative tumor cells more sensitive to CAR-T cells expressing TRAIL-mediated apoptosis markers. In the case of systemic diseases, low-dose radiation can effectively sensitize tumor cells and kill tumors with a lower dose of CAR-T cells, which potentially reduces the risk of CRS and improves efficacy. 102
Other Strategies
In addition to the treatment methods mentioned above, additional strategies are being envisaged or developed. For example, a split, universal, and programable (SUPRA) CAR system that connects CAR-T cells and tumor cells by splitting the antigen targeting domain and T-cell signaling unit has been developed and gives CAR-T cells the ability to recognize multiple antigens. These features are useful to combat relapse, mitigate overactivation, and enhance specificity. 103 Bispecific T-cell engager (BiTE) antibodies are artificial antibodies containing 2 specific antigen-binding sites that can bridge the gap between target cells and functional molecules (cells) and stimulate a directed immune response. 104 The CD19 BiTE blinatumomab is related to the mechanism of tumor evasion and antigen-negative relapse. 105
Conclusion
CAR-T cells have achieved excellent results in the treatment of B-cell malignancies. A large number of clinical trials have shown that pediatric and adult patients with B-ALL treated with anti-CD19 CAR-T cells attained a CR rate of approximately 90%. Although the overall effect is significant, some patients still relapse after treatment. Cases of relapse are divided into antigen-positive relapse and antigen-negative relapse according to the presence of antigen. Antigen-positive relapse is usually correlated with the strength and persistence of CAR-T cells. The source of leukocyte material used during CAR preparation can be improved, and the CAR structure can be modified to obtain more functional and durable CAR-T cells to reduce the possibility of antigen-positive relapse. In comparison, relapse caused by antigen escape is a substantial challenge. Only by overcoming this challenge can the clinical treatment effect be improved. The mechanisms of antigen escape, including reduction of antigen density, gene mutation, and lineage switching, are complex and dynamic. For the currently known antigen escape mechanisms, we can take corresponding countermeasures. For example, targeting other tumor-specific antigens, administration of multitarget therapy, modification of the CAR structure, combination with other small molecules, and combination with radiotherapy have achieved good clinical effects.
Unfortunately, the currently available methods cannot determine whether patients are at risk of antigen escape before CAR-T cell treatment. The antitumor effect of CAR-T cells is mainly mediated by perforin/granzyme. Upadhyay et al 106 found that CAR-T cells can mediate bystander killing of tumor cells whose target antigen is not expressed via the Fas/FasL pathway, and Fas expression can also predict the long-term prognosis of patients treated with CAR-T cells. Researchers have also demonstrated that T cells secrete IFN-γ, which can upregulate the expression of Fas in tumor cells. The currently known Fas-mediated cell death sensitizers GHDAC inhibitors and SMAC mimics can also enhance T-cell-mediated killing, which is independent of antigen expression. The combination of such Fas-related small molecule regulatory preparations and CAR-T cells may prevent the relapse caused by antigen escape to a certain extent.
For patients with initial intratumoral heterogeneity and antigen-negative clones at initial diagnosis, corresponding treatments may help prevent antigen escape. 107 Hematopoietic stem cell transplantation (HCT) is currently the only means to cure hematological malignancies. Therefore, some scholars believe that allogeneic HCT (allo-HCT) should be carried out immediately after CAR-T cell treatment is administered. 108 However, due to the lack of strong medical evidence, there are no unified guidelines for whether or when transplantation should be administered.
In conclusion, it is hoped that shortly, the emergence of additional control strategies will make CAR-T cell therapy a more effective and safer treatment.
Acknowledgments
DNX was a major contributor in writing the manuscript. XJ have made substantial contributions to the conception. MZ and RS have drafted the work. XX, JXW, and XMZ helped proposed some constructive suggestions. All authors contributed to the article and approved the submitted version.
Abbreviations
- BAFF-R
B-cell activating factor receptor
- B-ALL
B-cell acute lymphoblastic leukemia
- BiTE
bispecific T-cell engager
- CAR
chimeric antigen receptor
- CR/CRi
complete response/complete remission with incomplete hematologic recovery
- CRS
cytokine release syndrome
- CLL
chronic lymphocytic leukemia
- DLBCL
diffuse large B-cell lymphoma
- HCT
hematopoietic stem cell transplantation
- ICIs
immune checkpoint inhibitors
- Lck
lymphocyte-specific tyrosine kinase
- LSCs
leukemia stem cells
- MDSCs
myeloid-derived suppressor cells
- MHC
major histocompatibility complex
- MLL
mixed-lineage leukemia
- MRD
minimal residual disease
- NHL
non-Hodgkin's lymphoma
- ORR
objective response rate
- OS
overall survival
- R/R
refractory and/or relapsed
- scFV
single-chain variable fragment
- TCR
T-cell receptor
- TNF
tumor necrosis factor
- TRAIL
TNF-related apoptosis-inducing ligand
- TSLPR
thymic stromal lymphopoietin receptor
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the General Project of National Natural Science Foundation of China (81970180 to MZ), and the Key Science and Technology Support Project of Tianjin Science and Technology Bureau (20YFZCSY00800), and the Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK-056B), as well as Tianjin First Central Hospital.
ORCID iD: Danni Xie https://orcid.org/0000-0001-5453-0363
References
- 1.Stoiber S, Cadilha BL, Benmebarek MR, Lesch S, Endres S, Kobold S. Limitations in the design of chimeric antigen receptors for cancer therapy. Cells. 2019;8(5):472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.June CH, Maus MV, Plesa G, et al. Engineered T cells for cancer therapy. Cancer Immunol Immunother. 2014;63(9):969-975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18(4):843-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang J, Jensen M, Lin Y, et al. Optimizing adoptive polyclonal T cell immunotherapy of lymphomas, using a chimeric T cell receptor possessing CD28 and CD137 costimulatory domains. Hum Gene Ther. 2007;18(8):712-725. [DOI] [PubMed] [Google Scholar]
- 5.Luo Y, Song G, Liang S, Li F, Liu K. Research advances in chimeric antigen receptor-modified T-cell therapy (Review). Exp Ther Med. 2021;21(5):484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang R, Li X, He Y, et al. Recent advances in CAR-T cell engineering. J Hematol Oncol. 2020;13(1):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koneru M, Purdon TJ, Spriggs D, Koneru S, Brentjens RJ. IL-12 secreting tumor-targeted chimeric antigen receptor T cells eradicate ovarian tumors in vivo. Oncoimmunology. 2015;4(3):e994446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y, Sun C, Landoni E, Metelitsa L, Dotti G, Savoldo B. Eradication of neuroblastoma by T cells redirected with an optimized GD2-specific chimeric antigen receptor and interleukin-15. Clin Cancer Res. 2019;25(9):2915-2924. [DOI] [PubMed] [Google Scholar]
- 9.Park JH, Palomba ML, Batlevi CL, et al. A phase I first-in-human clinical trial of CD19-targeted 19–28z/4-1BBL “armored” CAR T cells in patients with relapsed or refractory NHL and CLL including richter’s transformation. Blood. 2018;132(Supplement 1):224. [Google Scholar]
- 10.Schuster SJ, Bishop M, Tam C, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45-56. [DOI] [PubMed] [Google Scholar]
- 11.Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019;20(1):31-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang M, Munoz J, Goy A, et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2020;382(14):1331-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839-852. [DOI] [PubMed] [Google Scholar]
- 14.Munshi NC, Anderson LD, Jr., Shah N, et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med. 2021;384(8):705-716. [DOI] [PubMed] [Google Scholar]
- 15.Berdeja JG, Madduri D, Usmani SZ, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet. 2021;398(10297):314-324. [DOI] [PubMed] [Google Scholar]
- 16.Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368(16):1509-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee D, Kochenderfer J, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385(9967):517-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gardner RA, Finney O, Annesley C, et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood. 2017;129(25):3322-3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang T, Cao L, Xie J, et al. Efficiency of CD19 chimeric antigen receptor-modified T cells for treatment of B cell malignancies in phase I clinical trials: a meta-analysis. Oncotarget. 2015;6(32):33961-33971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maude SL, Laetsch T, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mostafa Kamel Y. CAR-T Therapy, the End of a chapter or the beginning of a new one? Cancers (Basel). 2021;13(4):853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orlando EJ, Han X, Tribouley C, et al. Genetic mechanisms of target antigen loss in CAR19 therapy of acute lymphoblastic leukemia. Nat Med. 2018;24(10):1504-1506. [DOI] [PubMed] [Google Scholar]
- 24.Shah NN, Fry TJ. Mechanisms of resistance to CAR T cell therapy. Nat Rev Clin Oncol. 2019;16(6):372-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Das RK, Storm J, Barrett DM. T cell dysfunction in pediatric cancer patients at diagnosis and after chemotherapy can limit chimeric antigen receptor potential. Cancer Res. 2018;78(13):1631. [Google Scholar]
- 26.Lei X, Lei Y, Li JK, et al. Immune cells within the tumor microenvironment: biological functions and roles in cancer immunotherapy. Cancer Lett. 2020;470:126-133. [DOI] [PubMed] [Google Scholar]
- 27.Watanabe K, Kuramitsu S, Posey A, June C. Expanding the therapeutic window for CAR T cell therapy in solid tumors: the knowns and unknowns of CAR T cell biology. Front Immunol. 2018;9:2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hatic H, Sampat D, Goyal G. Immune checkpoint inhibitors in lymphoma: challenges and opportunities. Ann Transl Med. 2021;9(12):1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zolov S, Rietberg S, Bonifant C. Programmed cell death protein 1 activation preferentially inhibits CD28.CAR-T cells. Cytotherapy. 2018;20(10):1259-1266. [DOI] [PubMed] [Google Scholar]
- 30.Levine BL, Miskin J, Wonnacott K, Keir C. Global manufacturing of CAR T cell therapy. Mol Ther Methods Clin Dev. 2017;4:92-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Egri N, Ortiz de Landazuri I, San Bartolome C, Ortega JR, Espanol-Rego M, Juan M. CART Manufacturing process and reasons for academy-pharma collaboration. Immunol Lett. 2020;217:39-48. [DOI] [PubMed] [Google Scholar]
- 32.Jin X, Lu W, Zhang M, et al. Infection temperature affects the phenotype and function of chimeric antigen receptor T cells produced via lentiviral technology. Front Immunol. 2021;12:638907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baust JG, Snyder KK, Van Buskirk R, Baust JM. Integrating molecular control to improve cryopreservation outcome. Biopreserv Biobank. 2017;15(2):134-141. [DOI] [PubMed] [Google Scholar]
- 34.Golab K, Grose R, Placencia V, et al. Cell banking for regulatory T cell-based therapy: strategies to overcome the impact of cryopreservation on the treg viability and phenotype. Oncotarget. 2018;9(11):9728-9740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shah NN, Zhu FL, Schneider D, et al. Fresh versus cryopreserved/thawed bispecific anti-CD19/CD20 CAR-T cells for relapsed, refractory non-Hodgkin lymphoma. Blood. 2019;134(Supplement 1):4465. [Google Scholar]
- 36.Simon AK, Hollander GA, McMichael A. Evolution of the immune system in humans from infancy to old age. Proc Biol Sci. 2015;282(1821):20143085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh N, Perazzelli J, Grupp SA, Barrett DM. Early memory phenotypes drive T cell proliferation in patients with pediatric malignancies. Sci Transl Med. 2016;8(320):320ra3. [DOI] [PubMed] [Google Scholar]
- 38.Meyran D, Terry RL, Zhu JJ, et al. Early-phenotype CAR-T cells for the treatment of pediatric cancers. Ann Oncol. 2021;32(11):1366-1380. [DOI] [PubMed] [Google Scholar]
- 39.Gardner R, Finney O, Brakke H, et al. Starting T cell and cell product phenotype are associated with durable remission of leukemia following CD19 CAR-T cell immunotherapy. Blood. 2018;132(Supplement 1):4022. [Google Scholar]
- 40.Turtle CJ, Hanafi LA, Berger C, et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest. 2016;126(6):2123-2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park JH, Riviere I, Gonen M, et al. Long-Term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med. 2018;378(5):449-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kawalekar OU, O’Connor RS, Fraietta JA, et al. Distinct signaling of coreceptors regulates specific metabolism pathways and impacts memory development in CAR T cells. Immunity. 2016;44(2):380-390. [DOI] [PubMed] [Google Scholar]
- 43.Zhang M, Jin X, Sun R, et al. Optimization of metabolism to improve efficacy during CAR-T cell manufacturing. J Transl Med. 2021;19(1):499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Teijeira A, Garasa S, Etxeberria I, Gato-Canas M, Melero I, Delgoffe GM. Metabolic consequences of T-cell costimulation in anticancer immunity. Cancer Immunol Res. 2019;7(10):1564-1569. [DOI] [PubMed] [Google Scholar]
- 45.Weinkove R, George P, Dasyam N, McLellan AD. Selecting costimulatory domains for chimeric antigen receptors: functional and clinical considerations. Clin Transl Immunology. 2019;8(5):e1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salter AI, Ivey RG, Kennedy JJ, et al. Phosphoproteomic analysis of chimeric antigen receptor signaling reveals kinetic and quantitative differences that affect cell function. Sci Signal. 2018;11(544):eaat6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun C, Shou P, Du H, et al. THEMIS-SHP1 Recruitment by 4-1BB tunes LCK-mediated priming of chimeric antigen receptor-redirected T cells. Cancer Cell. 2020;37(2):216-225. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li G, Boucher JC, Kotani H, et al. 4-1BB Enhancement of CAR T function requires NF-kappaB and TRAFs. JCI Insight. 2018;3(18):e121322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ajina A, Maher J. Strategies to address chimeric antigen receptor tonic signaling. Mol Cancer Ther. 2018;17(9):1795-1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cao J, Wang G, Cheng H, et al. Potent anti-leukemia activities of humanized CD19-targeted chimeric antigen receptor T (CAR-T) cells in patients with relapsed/refractory acute lymphoblastic leukemia. Am J Hematol. 2018;93(7):851-858. [DOI] [PubMed] [Google Scholar]
- 51.Li X, Chen W. Mechanisms of failure of chimeric antigen receptor T-cell therapy. Curr Opin Hematol. 2019;26(6):427-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ramakrishna S, Highfill SL, Walsh Z, et al. Modulation of target antigen density improves CAR T-cell functionality and persistence. Clin Cancer Res. 2019;25(17):5329-5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gauthier J, Bezerra E, Hirayama A, et al. Factors associated with outcomes after a second CD19-targeted CAR T-cell infusion for refractory B-cell malignancies. Blood. 2021;137(3):323-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Palomba ML, Jun MP, Lymp J, et al. Postinfusion monitoring costs by site of care for patients with relapsed/refractory large B-cell lymphoma receiving third- or later-line treatment with lisocabtagene maraleucel in the TRANSCEND NHL 001 and OUTREACH trials. Leukemia Lymphoma. 2021;62(9):2169-2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Y, Zhong K, Ke J, et al. Combined 4-1BB and ICOS co-stimulation improves anti-tumor efficacy and persistence of dual anti-CD19/CD20 chimeric antigen receptor T cells. Cytotherapy. 2021;23(8):715-723. [DOI] [PubMed] [Google Scholar]
- 56.Safarzadeh Kozani P, Safarzadeh Kozani P, O’Connor RS. In like a lamb; out like a lion: marching CAR T cells toward enhanced efficacy in B-ALL. Mol Cancer Ther. 2021;20(7):1223-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qian L, Li D, Ma L, et al. The novel anti-CD19 chimeric antigen receptors with humanized scFv (single-chain variable fragment) trigger leukemia cell killing. Cell Immunol. 2016;304–305:49-54. [DOI] [PubMed] [Google Scholar]
- 58.Sommermeyer D, Hill T, Shamah S, et al. Fully human CD19-specific chimeric antigen receptors for T-cell therapy. Leukemia. 2017;31(10):2191-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mirzaei HR, Jamali A, Jafarzadeh L, et al. Construction and functional characterization of a fully human anti-CD19 chimeric antigen receptor (huCAR)-expressing primary human T cells. J Cell Physiol. 2019;234(6):9207-9215. [DOI] [PubMed] [Google Scholar]
- 60.Song W, Zhang M. Use of CAR-T cell therapy, PD-1 blockade, and their combination for the treatment of hematological malignancies. Clin Immunol. 2020;214:108382. [DOI] [PubMed] [Google Scholar]
- 61.Wang J, Deng Q, Jiang YY, et al. CAR-T 19 combined with reduced-dose PD-1 blockade therapy for treatment of refractory follicular lymphoma: a case report. Oncol Lett. 2019;18(5):4415-4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cao Y, Lu W, Sun R, et al. Anti-CD19 chimeric antigen receptor T cells in combination with nivolumab are safe and effective against relapsed/refractory B-cell non-Hodgkin lymphoma. Front Oncol. 2019;9:767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu X, Zhang Y, Li K, et al. A novel dominant-negative PD-1 armored anti-CD19 CAR T cell is safe and effective against refractory/relapsed B cell lymphoma. Transl Oncol. 2021;14(7):101085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wei J, Luo C, Wang Y, et al. PD-1 silencing impairs the anti-tumor function of chimeric antigen receptor modified T cells by inhibiting proliferation activity. J Immunother Cancer. 2019;7(1):209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yu H, Sotillo E, Harrington C, et al. Repeated loss of target surface antigen after immunotherapy in primary mediastinal large B cell lymphoma. Am J Hematol. 2017;92(1):E11-E13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zheng S, Asnani M, Thomas-Tikhonenko A. Escape from ALL-CARTaz: leukemia immunoediting in the age of chimeric antigen receptors. Cancer J. 2019;25(3):217-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hamieh M, Dobrin A, Cabriolu A, et al. CAR T cell trogocytosis and cooperative killing regulate tumour antigen escape. Nature. 2019;568(7750):112+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fry TJ, Shah NN, Orentas RJ, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med. 2018;24(1):20-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bowler E, Oltean S. Alternative splicing in angiogenesis. Int J Mol Sci. 2019;20(9):2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sotillo E, Barrett DM, Black KL, et al. Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov. 2015;5(12):1282-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fischer J, Paret C, El Malki K, et al. CD19 Isoforms enabling resistance to CART-19 immunotherapy are expressed in B-ALL patients at initial diagnosis. J Immunother. 2017;40(5):187-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gardner R, Wu D, Cherian S, et al. Acquisition of a CD19-negative myeloid phenotype allows immune escape of MLL-rearranged B-ALL from CD19 CAR-T-cell therapy. Blood. 2016;127(20):2406-2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liao W, Kohler ME, Fry T, Ernst P. Does lineage plasticity enable escape from CAR-T cell therapy? Lessons from MLL-r leukemia. Exp Hematol. 2021;103:73–74. [DOI] [PubMed] [Google Scholar]
- 74.Rayes A, McMasters R, O’Brien M. Lineage switch in MLL-rearranged infant leukemia following CD19-directed therapy. Pediatr Blood Cancer. 2016;63(6):1113-1115. [DOI] [PubMed] [Google Scholar]
- 75.Jacoby E, Nguyen S, Fountaine T, et al. CD19 CAR immune pressure induces B-precursor acute lymphoblastic leukaemia lineage switch exposing inherent leukaemic plasticity. Nat Commun. 2016;7:12320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Turtle CJ, Berger C, Sommermeyer D, et al. Anti-CD19 chimeric antigen receptor-modified T cell therapy for B cell non-Hodgkin lymphoma and chronic lymphocytic leukemia: fludarabine and cyclophosphamide lymphodepletion improves in vivo expansion and persistence of CAR-T cells and clinical outcomes. Blood. 2015;126(23):184. [Google Scholar]
- 77.Ruella M, Xu J, Barrett DM, et al. Induction of resistance to chimeric antigen receptor T cell therapy by transduction of a single leukemic B cell. Nat Med. 2018;24(10):1499.–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Seita A, Nakaoka H, Okura R, Wakamoto Y. Intrinsic growth heterogeneity of mouse leukemia cells underlies differential susceptibility to a growth-inhibiting anticancer drug. Plos One. 2021;16(2):e0236534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ruella M, Barrett DM, Kenderian SS, et al. Dual CD19 and CD123 targeting prevents antigen-loss relapses after CD19-directed immunotherapies. J Clin Invest. 2016;126(10):3814-3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pan J, Niu Q, Deng B, et al. CD22 CAR T-cell therapy in refractory or relapsed B acute lymphoblastic leukemia. Leukemia. 2019;33(12):2854-2866. [DOI] [PubMed] [Google Scholar]
- 81.Adeel K, Fergusson N, Shorr R, Atkins H, Hay K. Efficacy and safety of CD22 chimeric antigen receptor (CAR) T cell therapy in patients with B cell malignancies: a protocol for a systematic review and meta-analysis. Syst Rev. 2021;10(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhu H, Deng H, Mu J, Lyu C, Jiang Y, Deng Q. Anti-CD22 CAR-T cell therapy as a salvage treatment in B cell malignancies refractory or relapsed after anti-CD19 CAR-T therapy. Onco Targets Ther. 2021;14:4023-4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang W, Wang Y, Guo Y, et al. Treatment of CD20-directed chimeric antigen receptor-modified T cells in patients with relapsed or refractory B-cell non-Hodgkin lymphoma: an early phase IIa trial report. Signal Transduction Targeted Ther. 2016;1:16002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Qin H, Cho M, Haso W, et al. Eradication of B-ALL using chimeric antigen receptor-expressing T cells targeting the TSLPR oncoprotein. Blood. 2015;126(5):629-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Qin H, Dong Z, Wang X, et al. CAR T cells targeting BAFF-R can overcome CD19 antigen loss in B cell malignancies. Sci Transl Med. 2019;11(511):eaaw9414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wei J, Han X, Bo J, Han W. Target selection for CAR-T therapy. J Hematol Oncol. 2019;12(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Guo Z, Tu S, Yu S, et al. Preclinical and clinical advances in dual-target chimeric antigen receptor therapy for hematological malignancies. Cancer Sci. 2021;112(4):1357-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hua J, Qian W, Wu X, et al. Sequential infusion of anti-CD22 and anti-CD19 chimeric antigen receptor T cells for a pediatric ph-like B-ALL patient that relapsed after CART-cell and haplo-HSCT therapy: a case report and review of literature. Onco Targets Ther. 2020;13:2311-2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dai H, Wu Z, Jia H, et al. Bispecific CAR-T cells targeting both CD19 and CD22 for therapy of adults with relapsed or refractory B-cell acute lymphoblastic leukemia. J Hematol Oncol. 2020;13(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wei G, Zhang Y, Zhao H, et al. CD19/CD22 Dual-Targeted CAR T-cell therapy for relapsed/refractory aggressive B-cell lymphoma: a safety and efficacy study. Cancer Immunol Res. 2021;9(9):1061-1070. [DOI] [PubMed] [Google Scholar]
- 91.Testa U, Pelosi E, Castelli G. CD123 As a therapeutic target in the treatment of hematological malignancies. Cancers (Basel). 2019;11(9):1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Aldoss I, Clark M, Song JY, Pullarkat V. Targeting the alpha subunit of IL-3 receptor (CD123) in patients with acute leukemia. Hum Vaccin Immunother. 2020;16(10):2341-2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yanagisawa B, Perkins B, Karantanos T, et al. Expression of putative leukemia stem cell targets in genetically-defined acute myeloid leukemia subtypes. Leuk Res. 2020;99:106477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Qin H, Edwards JP, Zaritskaya L, et al. Chimeric antigen receptors incorporating D domains targeting CD123 direct potent mono- and bi-specific antitumor activity of T cells. Mol Ther. 2019;27(7):1262-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu J, Chen Z, Li Y, Zhao W, Wu J, Zhang Z. PD-1/PD-L1 checkpoint inhibitors in tumor immunotherapy. Front Pharmacol. 2021;12:731798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang Z, Wu X. Study and analysis of antitumor resistance mechanism of PD1/PD-L1 immune checkpoint blocker. Cancer Med. 2020;9(21):8086-8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jacobson CA, Westin JR, Miklos DB, et al. Phase 1/2 primary analysis of ZUMA-6: axicabtagene ciloleucel (Axi-Cel) in combination with atezolizumab (Atezo) for the treatment of patients (Pts) with refractory diffuse large B cell lymphoma (DLBCL). Cancer Res. 2020;80(16):CT055. [Google Scholar]
- 98.Ledererova A, Dostalova L, Kozlova V, et al. Hypermethylation of CD19 promoter enables antigen-negative escape to CART-19 in vivo and in vitro. J Immunother Cancer. 2021;9(8):e002352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bockel S, Durand B, Deutsch E. Combining radiation therapy and cancer immune therapies: from preclinical findings to clinical applications. Cancer Radiother. 2018;22(6–7):567-580. [DOI] [PubMed] [Google Scholar]
- 100.Supplitt S, Bartosik W, Kotowski K, et al. Car T-cell therapy as an innovative approach in cancer immunotherapy. Postepy Biol Komorki. 2019;46(2):159-171. [Google Scholar]
- 101.Singh D, Tewari M, Singh S, Narayan G. Revisiting the role of TRAIL/TRAIL-R in cancer biology and therapy. Future Oncol. 2021;17(5):581-596. [DOI] [PubMed] [Google Scholar]
- 102.DeSelm C, Palomba ML, Yahalom J, et al. Low-Dose radiation conditioning enables CAR T cells to mitigate antigen Escape. Molecular Therapy. 2018;26(11):2542-2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cho JH, Collins JJ, Wong WW. Universal chimeric antigen receptors for multiplexed and logical control of T cell responses. Cell. 2018;173(6):1426-1438. e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Patel A, Oluwole O, Savani B, Dholaria B. Taking a BiTE out of the CAR T space race. Br J Haematol. 2021;195(5):689-697. [DOI] [PubMed] [Google Scholar]
- 105.Bouziana S, Bouzianas D. Anti-CD19 CAR-T cells: digging in the dark side of the golden therapy. Crit Rev Oncol Hematol. 2021;157:103096. [DOI] [PubMed] [Google Scholar]
- 106.Upadhyay R, Boiarsky JA, Pantsulaia G, et al. A critical role for fas-mediated off-target tumor killing in T-cell immunotherapy. Cancer Discov. 2021;11(3):599-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kansagra AJ, Frey NV, Bar M, et al. Clinical utilization of Chimeric Antigen Receptor T-cells (CAR-T) in B-cell acute lymphoblastic leukemia (ALL)-an expert opinion from the European Society for Blood and Marrow Transplantation (EBMT) and the American Society for Blood and Marrow Transplantation (ASBMT). Bone Marrow Transpl. 2019;54(11):1868-1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bouziana S, Bouzianas D. Exploring the dilemma of allogeneic hematopoietic cell transplantation after chimeric antigen receptor T cell therapy: to transplant or not? Biol Blood Marrow Transplant. 2020;26(8):e183-e191. [DOI] [PubMed] [Google Scholar]