Highlights
-
•
Inflammatory myofibroblastic tumors can mimic leiomyosarcoma histologically.
-
•
Highlights the role of molecular testing in the diagnosis and management of uterine mesenchymal tumors
-
•
Anaplastic lymphoma kinase (ALK) is a receptor tyrosine kinase involved in cellular growth.
-
•
Alectinib is one of several ALK inhibitors FDA-approved for patients with ALK-fusion positive lung cancers
-
•
Supports the paradigm shift toward developing molecularly targeted therapies rather than disease site-specific treatments
Keywords: Case report, Uterine inflammatory myofibroblastic tumor, Uterine leiomyosarcoma, ALK-inhibitor, ALK fusion, TNS1-ALK fusion
Abstract
Soft tissue sarcomas encompass a wide range of histologic subtypes with varied clinical implications. The incorporation of comprehensive genetic profiling into clinical practice is refining our ability to make these distinctions in diagnosis to better reflect prognosis and clinical behavior of a tumor. In this report, we describe a case of recurrent inflammatory myofibroblastic tumor (IMT) of the uterus, initially diagnosed and managed as leiomyosarcoma. At the time of recurrence, the patient was found to have a TNS1-ALK rearrangement and was treated successfully with alectinib, a second-generation anaplastic lymphoma kinase (ALK)-inhibitor. She had a complete response by imaging six months after initiation of alectinib and remains without evidence of disease at 36 months follow-up. Pathology review in the setting of her known ALK fusion and the 2020 update to the World Health Organization Classification of Female Genital Tumors led to a change in diagnosis from leiomyosarcoma to IMT. Our case highlights the role of molecular testing in the diagnosis and management of uterine mesenchymal tumors and the efficacy of alectinib in this ALK-rearranged recurrent IMT of the uterus. Care must be taken to differentiate between IMT and other uterine mesenchymal tumors as this distinction can impact prognosis and management. Furthermore, this case adds to the growing body of evidence supporting the paradigm shift toward developing molecularly targeted therapies rather than disease site-specific treatments, especially in cases of recurrence as recommended by the National Comprehensive Cancer Network.
1. Introduction
Previously, nearly all uterine sarcomas were thought to represent leiomyosarcoma, low-grade endometrial stromal sarcoma, undifferentiated sarcoma, or ‘heterologous’ sarcomas such as rhabdomyosarcoma (Abu-Rustum, 2022, Fletcher, 2021). In recent years, additional histologic subtypes have been described and were recognized in the 5th edition of World Health Organization (WHO) Classification of Female Genital Tumors published in 2020. A major feature of the updated classification compared to the prior edition is a greater emphasis on key molecular events with integration of morphological and molecular features of tumors into the diagnostic criteria (WHO Classification of Tumors Editorial Board, 2020, McCluggage et al., 2020).
Among the newly detailed tumor types is inflammatory myofibroblastic tumor (IMT), which has been a long-underdiagnosed entity. IMTs can be found in a wide range of anatomic locations with 75 % occurring in the abdominal cavity, particularly in the mesentery, greater omentum, and retroperitoneal space with remaining sites comprising of the areas of the head and neck, lungs, urinary bladder, central nervous system, and the female genital tract (Siemion et al., 2022). Following excision, approximately 10–25 % of patients develop local recurrence and < 5 % demonstrate metastatic disease. Histologically, these tumors are variably cellular, composed of myofibroblastic and fibroblastic spindle cells with loose myxoid, fascicular/compact or hyalinized growth patterns, variable (usually mild) cytologic atypia and mitotic rate, and accompanied by a prominent lymphoplasmacytic infiltrate (Fletcher, 2021).
Most IMTs overexpress anaplastic lymphoma kinase (ALK) protein by immunohistochemistry and are associated with ALK gene fusions (Abu-Rustum, 2022, McCluggage et al., 2020, Davare and Tognon, 2015). ALK is a receptor tyrosine kinase located on chromosome 2p23 which is involved in cellular growth (Davare and Tognon, 2015). ALK gene alterations can generate ALK fusion proteins which retain the ALK kinase domain sequence and become constitutively activated. ALK fusion proteins have been well-established in non-small cell lung cancer (NSCLC) (Davis et al., 2019). Approximately 50–60 % of IMTs harbor ALK-gene rearrangements compared to 2.4 % of reported leiomyosarcomas (Siemion et al., 2022, Davis et al., 2019). Reported gene fusions involved in IMT include TPM3-ALK, TPM4-ALK, CLTC-ALK, RANBP2-ALK, CARS-ALK, ATIC-ALK, ETV6-NTRK3, and TFG-ROS1 (Agulnik et al., 2022). Since the increased recognition of IMT, the National Comprehensive Cancer Network (NCCN) Guidelines for Soft Tissue Sarcomas recommend ALK-inhibitors as first line treatment of IMT with ALK fusions citing crizotinib, ceritinib, brigatinib, and lorlatinib for this indication (Agulnik et al., 2022). Alectinib (ALECENSA), a second generation ALK-inhibitor, is not listed in the guidelines for treatment of IMT but is one of several ALK-inhibitors that has been FDA-approved and is established as standard of care for patients with ALK fusion positive lung cancers along with crizotinib, lorlatinib and ceritinib (Davis et al., 2019).
Here, we present a case of uterine IMT that recurred with local pelvic and pulmonary metastatic disease. The patient was initially diagnosed with a uterine leiomyosarcoma and after recurring, progressed on multiple lines of chemotherapy. Tumor genomic profiling was performed on the original tumor and a TNS1-ALK rearrangement was detected, which ultimately prompted a pathology re-review of the original resection specimen and subsequently the diagnosis was revised to malignant IMT. She was started on alectinib, and subsequent imaging showed a robust response with regression of her tumor and a sustained remission of over 36 months.
2. Case
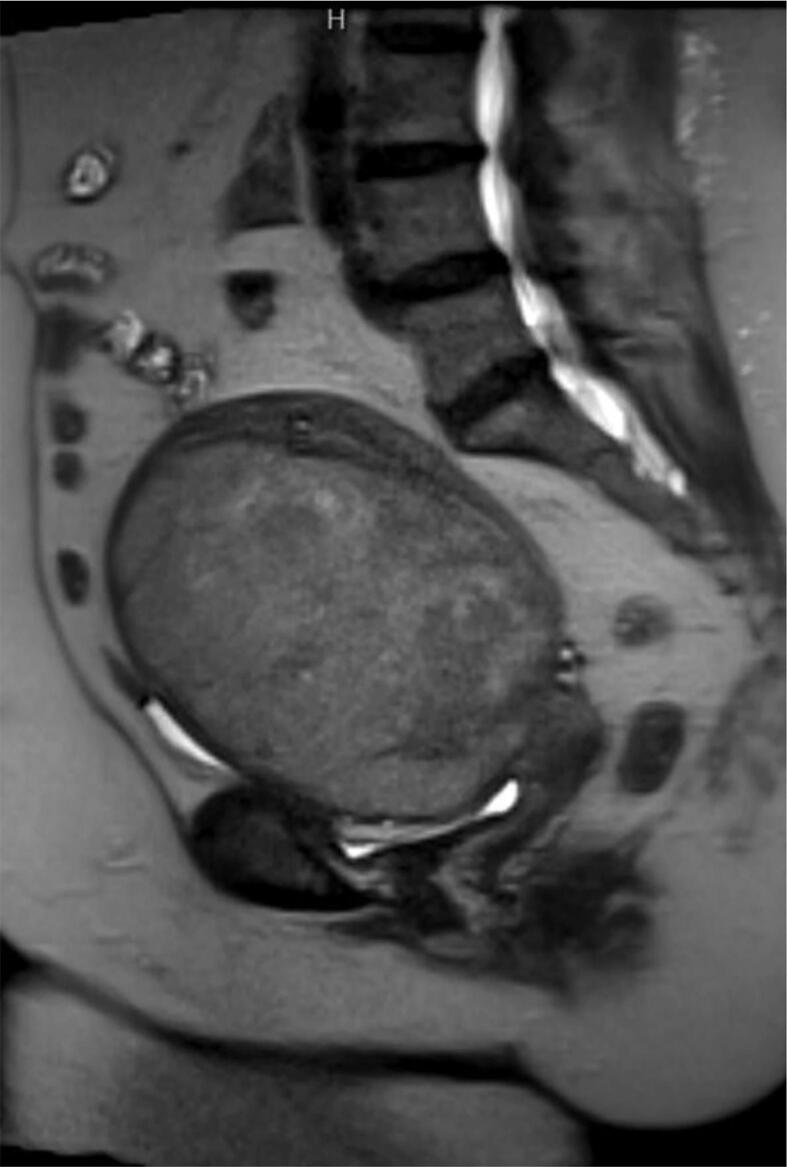
The patient was a 71-year-old postmenopausal woman who presented to her primary care physician’s office for pelvic pain and pressure. Transvaginal ultrasound revealed a presumed uterine leiomyoma measuring 19.5 × 12.3 × 9.8 cm. Magnetic resonance imaging (MRI) of the abdomen and pelvis confirmed a large 13 cm heterogeneous uterine mass with ill-defined margins suspicious for leiomyosarcoma (Fig. 1).
Fig. 1.
Preoperative MRI, showing Large 13 cm heterogeneous uterine mass with ill-defined margins, suspicious for leiomyosarcoma. No pelvic or inguinal lymphadenopathy.
No definite extension was noted beyond the uterine wall; however, the mass abutted the pelvic sidewalls and iliac vasculature with small collaterals within the pelvis and anterior abdomen. No pelvic or inguinal lymphadenopathy was seen. Computed tomography (CT) scan of the chest revealed no evidence of metastatic disease. She denied a history of postmenopausal bleeding, and a preoperative endometrial biopsy was not performed. She underwent an exploratory laparotomy, total abdominal hysterectomy with bilateral salpingo-oophorectomy. Intraoperative consultation on the uterine mass was interpreted as an atypical smooth muscle tumor. Final pathology was reported as leiomyosarcoma measuring 11.7 cm in greatest dimension with lymphovascular space invasion. The margins were free of tumor. Final pathology was consistent with stage 1B uterine leiomyosarcoma.
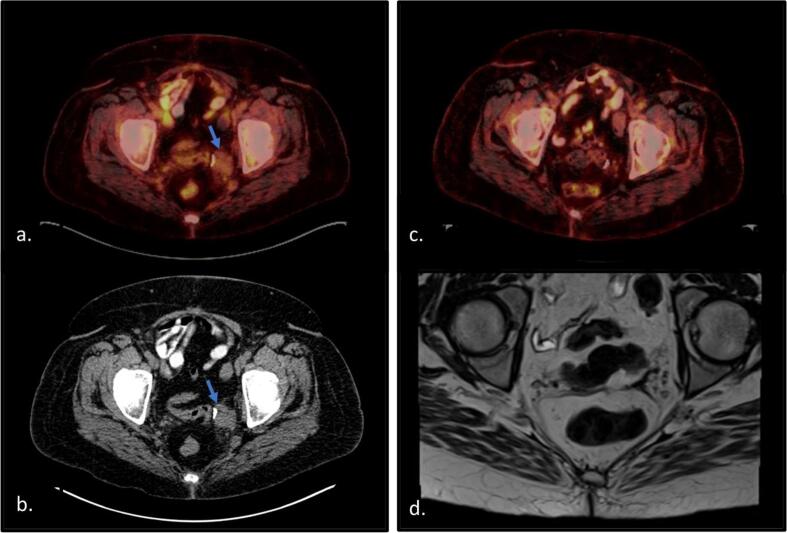
Postoperatively, the patient completed four cycles of adjuvant gemcitabine and docetaxel per the NCCN guidelines for management of leiomyosarcoma. Three years after completion of chemotherapy she was diagnosed with recurrent disease following a positron emission tomography (PET)-CT revealing hypermetabolic soft tissue lesions in the pelvic surgical bed and pulmonary nodules. Biopsy of the pelvic mass was reported as recurrent leiomyosarcoma. She was treated with six cycles of olaratumab (a PDGFR-alpha blocker) and doxorubicin. She initially had stable disease on treatment; however, repeat CT scan three months after completion of treatment showed progression of soft tissue implants in the pelvis (Fig. 2a and b).
Fig. 2.
PET CT and MRI imaging demonstrating recurrence and disease response to alectinib. a) PET CT showing FDG avid pelvic soft tissue lesion of biopsy proven recurrence (SUV 3.1). b) CT showing the 3.2 × 3 × 2.3 cm mass increased in size following second-line therapy for leiomyosarcoma. Additional soft tissue nodules were seen in at the vaginal cuff and in the right middle lobe of the lung and mediastinum, not pictured here. c) PET-CT showing disease response to alectinib. d) Given low FDG avidity of biopsy proven recurrence, MRI was recommended for best assessment of residual disease. MRI six months after initiation alectinib was without evidence of disease.
Tumor genomic profiling was performed on her recurrent pelvic tumor biopsy which showed TNS1-ALK rearrangement, CDKN2A/B loss, low tumor mutational burden (3Muts/Mb), and lack of microsatellite instability. No FDA approved therapies or clinical trials were identified for CDKN2A/B loss; however, ALK-inhibitors were identified treatment options in non-small cell lung cancer (NSCLC). After consultation with pharmacy and with an understanding that this was not a standard treatment for uterine leiomyosarcoma, she was started on alectinib. Repeat PET-CT ten weeks after starting treatment showed interval decrease in size and avidity of the pulmonary nodules and soft tissue masses within the pelvis with no evidence of new metabolically active disease. Imaging six months after treatment initiation showed a complete response with no residual disease (Fig. 2c and d).
A review of the patient’s initial pathology was performed following the latest 5th edition of WHO Classification of Female Genital Tumors, and in the context of her molecular testing showing an ALK fusion. The diagnosis was changed from leiomyosarcoma to IMT (Fig. 3).
Fig. 3.
Pathology. Uterine IMT with focally infiltrative borders (a), composed of intersecting fascicles of spindle cells (b) with moderate cytologic atypia, associated with lymphoplasmacytic infiltrate (d). Myxoid change is not conspicuous. Hematoxylin-eosin stain; original magnification: x100 (a, b), x200 (c, d).
The patient has remained on alectinib for a total of 36 months, which she has tolerated well. Her surveillance imaging has showed no evidence of recurrent disease through the time of this report.
3. Discussion
Advancements in genomic sequencing have allowed the discovery of novel oncogenic drivers and subsequent targeted therapies across multiple cancer types, markedly impacting treatment options and patient outcomes (Xia et al., 2021). Since the incorporation of tumor molecular testing and the latest edition of WHO Classification of Female Genital Tumors from 2020, uterine IMTs are being increasingly diagnosed and studied. Uterine sarcomas are uncommon neoplasms that account for 2–4 % of uterine malignancies. While leiomyosarcomas comprise 70 % of all malignant mesenchymal tumors of the uterus, they remain rare with an overall incidence of 0.4–0.9 in 100,000 women (Mas et al., 2019). The overall incidence of uterine IMT is not well established, although it is likely underdiagnosed due to being mistaken for other more common benign and malignant tumors including leiomyoma, leiomyosarcoma, and endometrial stromal sarcoma (Shukla and Mittal, 2019, Hensley et al., 2020). IMTs are most commonly benign but have a potential for extrauterine spread and/or recurrence. In a study of 23 cases of IMT, only 4 behaved in a malignant fashion as determined by the presence of extrauterine disease at diagnosis and/or recurrence (Bennett et al., 2020).
Several cases have been described where a previous diagnosis was revised after discovering an ALK fusion on molecular testing (Hensley et al., 2020, Kyi et al., 2021, Lee et al., 2019, Zhang et al., 2020, Testa et al., 2021). On review of the literature, this is the third reported case of uterine IMT with an aggressive clinical behavior containing a TNS1-ALK fusion specifically (Kyi et al., 2021, Lee et al., 2019). This is the second such case treated with alectinib. In 2021, Kyi et al described a case of uterine IMT containing TNS1-ALK fusion that was treated with alectinib as second line therapy for 12 months prior to disease progression (Kyi et al., 2021). There are also limited reports of leiomyosarcomas containing ALK fusions, but it cannot be discerned from the published manuscripts if IMT was considered in the differential diagnosis (Davis et al., 2019, Ross et al., 20172017). Of note, fascicular areas in uterine IMTs tend to be more cellular and often have less prominent inflammation compared with myxoid areas. Therefore, IMTs exhibiting a fascicular growth pattern exclusively or predominantly appear to closely resemble and mimic a smooth muscle neoplasm, as demonstrated in our case.
The distinction between leiomyosarcoma and IMT is meaningful in treatment planning. The preferred first-line systemic therapies for leiomyosarcoma include doxorubicin and gemcitabine/docetaxel, still reporting a high recurrence rate of approximately 50–70 % (Abu-Rustum, 2022). If a tumor is determined to be an IMT, these cytotoxic regimens are not recommended in the upfront setting or as first line in the recurrent setting when ALK fusions are present (Agulnik et al., 2022). Regardless of diagnosis, the presence of an ALK fusion provides an opportunity for a targeted treatment option with demonstrated clinical activity (Kyi et al., 2021, Testa et al., 2021, McKeage, 2015). The NCCN Guidelines for Uterine Neoplasms now recommend comprehensive genomic profiling with a validated assays including at least NTRK, MSI, and TMB as it is informative for pathologic characterization and identifying rare pan-tumor targeted therapy opportunities (Abu-Rustum, 2022). The NCCN Guidelines for Soft Tissue Sarcomas recommends that ALK fusion-containing IMTs regardless of disease site are treated with ALK-inhibitors as previously listed, but not including alectinib (Agulnik et al., 2022).
Alectinib is an orally available second generation ALK-inhibitor with antineoplastic activity. It binds to and inhibits ALK kinase, ALK fusion proteins, and the gatekeeper mutation ALKL1196M to disrupt ALK-mediated signaling with the overall function of inhibiting tumor cell growth in ALK-overexpressing tumor cells (Mok et al., 2020). Currently, it is used as first-line therapy for ALK-positive NSCLC with a 12-month event free survival rate of 68 % (Ross et al., 20172017). To date, ALK-inhibitors are only FDA approved to treat NSCLC (Abu-Rustum, 2022). The ALEX trial is a recent international, randomized, open-labeled phase 3 trial in which alectinib was compared to crizotinib, the previous standard of care ALK-inhibitor in patients with untreated, advanced ALK-positive NSCLC. They treated patients until disease progression, toxicity, withdrawal, or death reporting a median treatment duration of 28.1 months with alectinib compared to 10.8 months21. Common side effects include fatigue, hematuria, cough, joint pain and swelling. Toxicities requiring discontinuation include hemolytic anemia, symptomatic bradycardia, interstitial lung disease and CPK elevation greater than five times the upper limit of normal. Extrapolating from lung cancer data in the absence of studies regarding alectinib in uterine IMTs, we intend to continue treatment until the patient progresses or has toxicity with reassessment at the five-year mark. To date, she does not report any toxicities.
Our case highlights the role of molecular testing in the diagnosis and management of uterine mesenchymal tumors and the efficacy of alectinib in this ALK-rearranged recurrent IMT of the uterus. Clinicians need to be aware of this entity in the differential as it can impact prognosis and treatment planning. Furthermore, this case adds to the growing body of evidence supporting the paradigm shift toward developing molecularly targeted therapies based on individual tumor characteristics rather than solely relying on histology and disease site-specific treatments.
Informed consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
CRediT authorship contribution statement
Erica V. Carballo: Writing – original draft. Tra V. Pham: Writing – review & editing. Gulisa Turashvili: Writing – review & editing. Krisztina Hanley: Writing – review & editing. Kristen D. Starbuck: Writing – review & editing, Supervision. Jane L. Meisel: Writing – review & editing, Conceptualization, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abu-Rustum N.R. NCCN Clinical Practice Guidelines in Oncology Version 1.2022 Uterine Neoplasms. Natl Comprehensive Cancer Netw. 2022 [Google Scholar]
- Agulnik, M., Bui, M.M., Carr-Ascher, J., et al., 2022. NCCN Guidelines Version 2.2022 Soft Tissue Sarcoma. Accessed June 25. https://www.nccn.org/home/member-.
- Bennett J.A., Croce S., Pesci A., Niu N., Van de Vijver K., Burks E.J., Burandt E., Zannoni G.F., Rabban J.T., Oliva E. Inflammatory myofibroblastic tumor of the uterus: an immunohistochemical study of 23 cases. Am. J. Surg. Pathol. 2020;44(11):1441–1449. doi: 10.1097/PAS.0000000000001525. [DOI] [PubMed] [Google Scholar]
- Davare M.A., Tognon C.E. Detecting and targeting oncogenic fusion proteins in the genomic era. Biol. Cell. 2015;107(5):111–129. doi: 10.1111/BOC.201400096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis L.E., Nusser K.D., Przybyl J., et al. Discovery and characterization of recurrent, targetable ALK fusions in leiomyosarcoma. Mol. Cancer Res. 2019;17(3):676–685. doi: 10.1158/1541-7786.MCR-18-1075. [DOI] [PubMed] [Google Scholar]
- Fletcher, C.D.M. (Ed.), 2021. Tumors of Soft Tissue. In: Diagnostic Histopathology of Tumors, fifth ed. Elsevier, Inc. 1919-2001. Accessed June 25, 202. (Chapter 24) https://www-clinicalkey-com.proxy.library.emory.edu/#!/browse/book/3-s2.0-C20141049691.
- Hensley M.L., Chavan S.S., Solit D.B., et al. Genomic landscape of uterine sarcomas defined through prospective clinical sequencing. Clin. Cancer Res. 2020;26(14):3881–3888. doi: 10.1158/1078-0432.CCR-19-3959/15421/AM/GENOMIC-LANDSCAPE-OF-UTERINE-SARCOMAS-DEFINED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyi C., Friedman C.F., Mueller J.J., Benayed R., Ladanyi M., Arcila M., Yang S.R., Hensley M.L., Chiang S. Uterine mesenchymal tumors harboring ALK fusions and response to ALK-targeted therapy. Gynecol. Oncol Rep. 2021;37:100852. doi: 10.1016/j.gore.2021.100852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Singh A., Ali S.M., Lin D.I., Klempner S.J. TNS1-ALK fusion in a recurrent, metastatic uterine mesenchymal tumor originally diagnosed as leiomyosarcoma. Acta Med Acad. 2019;48(1):116–120. doi: 10.5644/AMA2006-124.248. [DOI] [PubMed] [Google Scholar]
- Mas A., Alonso R., Garrido-Gómez T., Escorcia P., Montero B., Jiménez-Almazán J., Martín J., Pellicer N., Monleón J., Simón C. The differential diagnoses of uterine leiomyomas and leiomyosarcomas using DNA and RNA sequencing. Am. J. Obstet. Gynecol. 2019;221(4):320.e1–320.e23. doi: 10.1016/j.ajog.2019.05.018. [DOI] [PubMed] [Google Scholar]
- McCluggage W.G., Singh N., Gilks C.B. Key changes to the World Health Organization (WHO) classification of female genital tumors introduced in the 5th edition (2020) Histopathology. 2022;80(5):762–778. doi: 10.1111/HIS.14609. [DOI] [PubMed] [Google Scholar]
- McKeage K. Alectinib: a review of its use in advanced ALK-rearranged non-small cell lung cancer. Drugs. 2015;75(1):75–82. doi: 10.1007/s40265-014-0329-y. [DOI] [PubMed] [Google Scholar]
- Mok T., Camidge D.R., Gadgeel S.M., Rosell R., Dziadziuszko R., Kim D.-W., Pérol M., Ou S.-H.-I., Ahn J.S., Shaw A.T., Bordogna W., Smoljanović V., Hilton M., Ruf T., Noé J., Peters S. Updated overall survival and final progression-free survival data for patients with treatment-naive advanced ALK-positive non-small-cell lung cancer in the ALEX study. Ann. Oncol. 2020;31(8):1056–1064. doi: 10.1016/j.annonc.2020.04.478. [DOI] [PubMed] [Google Scholar]
- Ross J.S., Ali S.M., Fasan O., et al. ALK Fusions in a wide variety of tumor types respond to anti-ALK targeted therapy. Oncologist. 2017;22(12):1444–1450. doi: 10.1634/THEONCOLOGIST.2016-0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla P.S., Mittal K. Inflammatory myofibroblastic tumor in female genital tract. Arch. Pathol. Lab. Med. 2019;143(1):122–129. doi: 10.5858/ARPA.2017-0575-RA. [DOI] [PubMed] [Google Scholar]
- Siemion K., Reszec-Gielazyn J., Kisluk J., Roszkowiak L., Zak J., Korzynska A. What do we know about inflammatory myofibroblastic tumors? - A systematic review. Adv. Med. Sci. 2022;67(1):129–138. doi: 10.1016/J.ADVMS.2022.02.002. [DOI] [PubMed] [Google Scholar]
- Testa S., Million L., Longacre T., Bui N. Uterine leiomyosarcoma with FN1-anaplastic lymphoma kinase fusion responsive to alectinib and lorlatinib. Case Rep. Oncol. 2021;14(2):812–819. doi: 10.1159/000516758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Classification of Tumors Editorial Board, ed. Female Genital Tumors: WHO Classification of Tumors, fifth ed., Vol 4. World Health Organization. Accessed June 25, 2022. https://publications.iarc.fr/Book-And-Report-Series/Who-Classification-Of-Tumours/Female-Genital-Tumours-2020.
- Xia P., Zhang L., Li P., Liu E., Li W., Zhang J., Li H., Su X., Jiang G. Molecular characteristics and clinical outcomes of complex ALK rearrangements identified by next-generation sequencing in non-small cell lung cancers. J. Transl. Med. 2021;19(1) doi: 10.1186/s12967-021-02982-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Wang Z., Zhuang R., et al. Efficacy and resistance of ALK inhibitors in two inflammatory myofibroblastic tumor patients with ALK fusions assessed by whole exome and RNA sequencing. Onco Targets Ther. 2020;13:10335–10342. doi: 10.2147/OTT.S270481. [DOI] [PMC free article] [PubMed] [Google Scholar]