Abstract
Endometrial damage is an important factor leading to infertility and traditional conventional treatments have limited efficacy. As an emerging technology in recent years, stem cell therapy has provided new hope for the treatment of this disease. By comparing the advantages of stem cells from different sources, it is believed that menstrual blood endometrial stem cells have a good application prospect as a new source of stem cells. However, the clinical utility of stem cells is still limited by issues such as colonization rates, long-term efficacy, tumor formation, and storage and transportation. This paper summarizes the mechanism by which stem cells repair endometrial damage and clarifies the material basis of their effects from four aspects: replacement of damaged sites, paracrine effects, interaction with growth factors, and other new targets. According to the pathological characteristics and treatment requirements of intrauterine adhesion (IUA), the research work to solve the above problems from the aspects of functional bioscaffold preparation and multi-functional platform construction is also summarized. From the perspective of scaffold materials and component functions, this review will provide a reference for comprehensively optimizing the clinical application of stem cells.
Keywords: IUA, Stem cell therapy, Endometrial injury, Scaffold material, Hydrogel
Graphical abstract
1. The significance and status of treatment of endometrial injury
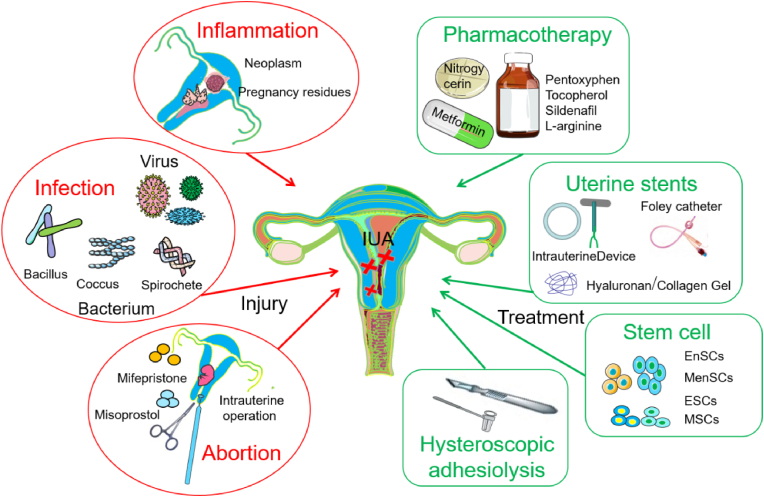
Endometriums one of the important components of the uterus, providing an essential internal environment for embryo implantation and pregnancy maintenance. Uterine cavity manipulation, inflammation and infection are risk factors for severe endometrial damage and intrauterine adhesion (IUA), which can directly lead to amenorrhea, infertility, abortion or other serious symptoms [[1], [2], [3]]. The mechanism by which IUA occurs is that the viability of endometrial stromal cells decreases or even undergoes apoptosis, which induces endometrial atrophy and the destruction of endometrial homeostasis [4]. The apoptosis signaling pathway activated by endometrial injury also inhibits endometrial angiogenesis, thereby hindering endometrial regeneration. During this process, damage to the basal lamina is usually irreversible and accompanied by fibrosis, manifested as Asherman syndrome, which means that only atrophic and inactive uterine glands and a small amount of stroma exist [5,6]. Clinically, re-epithelialization and revascularization of the exposed surface of the uterus are key factors for successful repair and remodeling of endometrium [[7], [8], [9]]. Anti-infection and inflammatory control are important environmental guarantees for optimizing endometrial neogenesis. Therefore, it is an urgent problem for gynecology to explore multifunctional therapies to repair endometrial injury, improve its microenvironment and tolerance state, and thus improve the pregnancy rate of infertility patients [10]. Intrauterine adhesion dissection is the preferred treatment method for IUA, but the re-adhesion rate after severe IUA can be as high as 62.5%. In addition to surgical treatment, solid barriers such as intrauterine devices often cause local inflammation, and simple semi-solid barriers such as hyaluronic acid hydrogels have limited repair effect. Metformin, nitroglycerin and other drugs may increase blood flow, but their efficacy is far from ideal [[11], [12], [13], [14]] (Fig. 1).
Fig. 1.
Mechanisms of endometrial injury and main treatment strategies.
Stem cell therapy, as an emerging and promising therapy in many fields, has been playing an irreplaceable role in treatment of various diseases such as brain injury and leukemia [15]. In the treatment of IUA, stem cells have also been widely used, and satisfactory results have been achieved in reducing endometrial fibrosis, repairing damaged endometrium, and improving uterine receptivity [16]. In vitro and in vivo experiments have shown that embryonic stem cells and mesenchymal stem cells have a great therapeutic effect on the IUA model [2,3]. However, these stem cells are difficult to obtain, which greatly limits their clinical application. It is well known that the normal endometrium of women of childbearing age usually undergoes approximately 400 cycles of proliferation, differentiation, shedding, and regeneration without scarring due to endometrial-intrinsic stem or progenitor cells that create new functions for blastocyst implantation. This process is the only example of scar-free repair in adult tissues, including inflammation, tissue remodeling, and proliferation and differentiation of endometrial stem cells [2]. In recent years, various stem cells present in the endometrium have been identified and characterized [[17], [18], [19]]. Among them, endometrial stem cells, especially non-traumatically available menstrual blood-derived stem cells (MenSCs), as a new type of stem cell population, have great potential in repairing endometrial injury caused by IUA due to their advantages of abundant sources, high proliferation rate, low immunogenicity and no ethical issue [4,5,20]. In this review, we will systematically analyze the advantages, mechanisms, and application strategies of stem cells in the treatment of IUA, so as to provide reference for promoting the clinical application of stem cell therapy for endometrial injury.
2. Comparison of the advantages of different sources of stem cells in the treatment of IUA
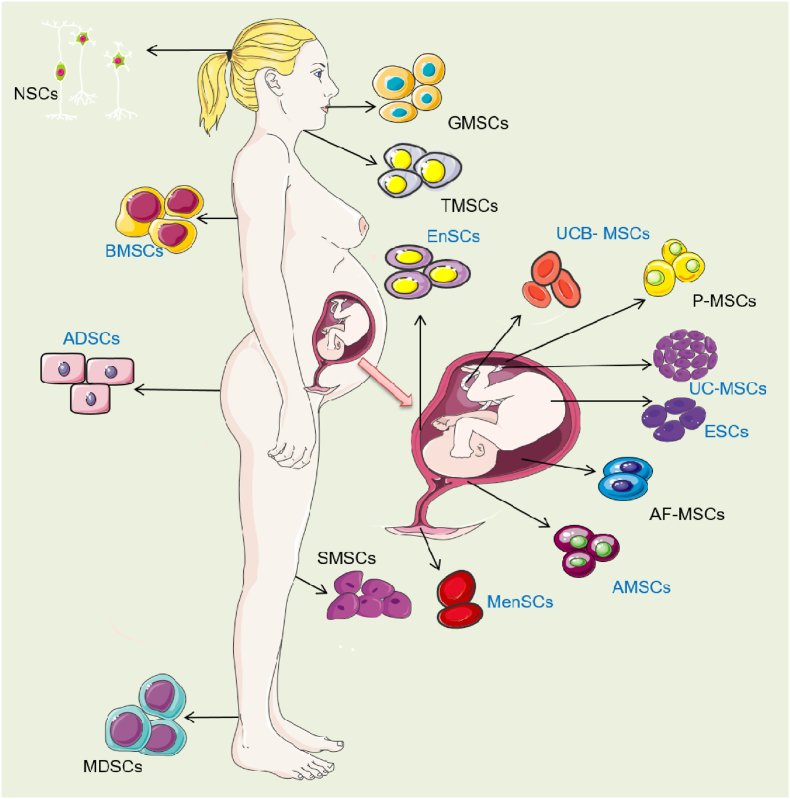
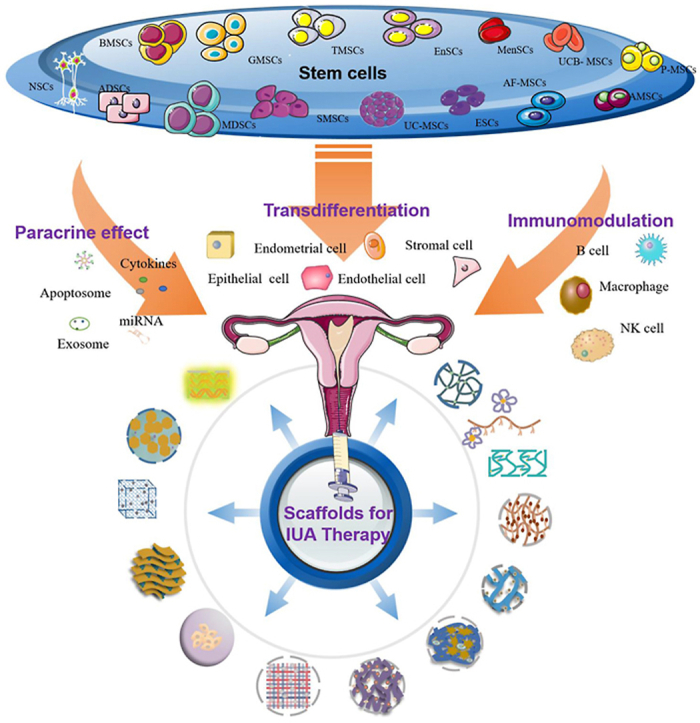
Stem cells are a class of cells with strong self-renewal ability and multi-directional proliferation and differentiation ability, so they can directly induce differentiation and replace damaged tissues to play an advantageous therapeutic role (Fig. 2). The comparison of stem cells from different sources and examples of application are shown in Table 1. Generally, stem cells can be divided into embryonic stem cells (ESCs) and adult stem cells (ASCs) according to their developmental sequence. At present, mesenchymal stem cells (MSCs) are the most widely used, which have been successfully isolated from bone marrow, adipose tissue, umbilical cord blood, menstrual blood, amniotic membrane and other tissues, and have been initially used in clinical practice.
Fig. 2.
Human derived stem cells. Stem cells which have been reported to treat endometrial injury were labeled with blue color. Neural stem cells (NSCs); Bone marrow derived mesenchymal stem cells (BMSCs); Adipose-derived stem cells (ADSCs); skeletal Muscle mesenchymal stem cells (MDSCs); Gingival mesenchymal stem cells (GMSCs); Tonsil mesenchymal stem cells (TMSCs); Synovium-derived mesenchymal stem cells (SMSCs); human Umbilical cord blood mesenchymal stem cells (UCB- MSCs); Endometrial mesenchymal stem cells (EnSCs); Menstrual blood derived mesenchymal stem cells (MenSCs); human Placental mesenchymal stem cells (P-MSCs); Umbilical cord mesenchymal stem cells (UC-MSCs); embryonic stem cells (ESCs); Amniotic fluid mesenchymal stem cells (AF-MSCs); human amniotic mesenchymal stromal cells (AMSCs).
Table 1.
Advantages and disadvantages of stem cells from different sources and their applications.
| Sources | Disease/Model | Applications | Advantages | Disadvantages | Ref |
|---|---|---|---|---|---|
| Human EnMSCs | Patients with thin endometrium | Increased thickness of endometrium and regenerative capacity. | Non-invasive harvesting procedure, easy expansion in vitro, and no ethical considerations. | Unclear the fate and long-term efficiency. | [21] |
| Human EnSCs | A woman with IUA | Increased endometrial thickness and pregnancy potential. | [22] | ||
| Human EnSCs | The female BALB/c nude mice with the ovarian cancer | Inhibit the epithelial ovarian cancer. | [23] | ||
| Human MenSCs | Infertile women with severe AS | Increased thickness of endometrium and pregnancies. | Non-invasive operation to the human body, easy to collect, and not involved in ethical issues. | Difficult preservation and high contamination rate. | [24] |
| Human MenSCs | Infertile women with refractory IUA | No adverse reaction and increased the endometrial thickness, 41.7% pregnancy rate. | [25] | ||
| Human MenSCs | The ESCs were wounded with mifepristone | In the treatment of endometrial injury: activated the AKT and p38 MAPK signaling pathways. | [26] | ||
| Human MenSCs |
The ESCs were fibrosis with TGFβ. | Inhibited myofibroblast differentiation of ESCs: activated Hippo/TAZ signal. | [27] | ||
| Human BMSCs | Human Asherman's syndrome (AS) and/or endometrial atrophy (EA) | Increased the volume and the thickness of endometrium, decreased intrauterine adhesion scores. | Sufficient sources; easy to obtain; great cell proliferation ability; no immune rejection. | Affected the psychology of both the donors and the recipients; Huge individual differences in the proliferation, survival, differentiation and paracrine capacity; Usually lead to infection after implantation. |
[28] |
| Human bone marrow-derived stem cells (BMDSCs) | Human with refractory AS or EA | Increased menstrual flow and pregnancies. | [29] | ||
| Rat BMSCs | Rat AS model | 70% conceived rate. | [30] | ||
| Human bone marrow mononuclear cells (BMNCs) | Patients with AS | Improved pregnancies and live births: downregulated ΔNp63 expression. | [31] | ||
| Human BMNCs | Women with AS | Restored endometrium. | [32] | ||
| Human UC-MSCs | Patient with IUA | Ten of the 26 patients had become pregnant, and eight of them had delivered live babies. | Lower immunogenicity, higher proliferation and self-renewal ability; extensive sources, no ethical disputes, no harm to the donor when obtaining the cells. | Lack of large animal data; few clinical applications. | [33] |
| Human UC-MSCs | Patients with IUA or cesarean scar diverticulum | Improved safety for poor healing after uterine injury. | [34] | ||
| Human UC-MSCs | Rat AS model | Increased blood supply; inhibited fibration; and restored the fertility. | [35] | ||
| Human ADSCs | Infertile women with severe AS | Increased menstrual flow and endometrial thickness, embryo transferred successfully. | Higher proliferative capacity and anti-aging ability; Greater secretion capacity; many obtaining ways and easy operation process; less limitation on ethics. | No standardized method for in vitro extraction; Activity decreases with age | [36] |
| Rat ADSCs | Rat AS model | Decreased inflammation and fibrosis and increased vascular proliferation. | [37] | ||
| Human perivascular stem cells (HPVSCs) | Mouse AS model | Ameliorated compromised uterine environments: facilitated HIF1α-dependent angiogenesis. | Relative higher purity; stronger osteogenic ability. | Harsh extraction conditions. | [38] |
| Human amniotic epithelial cells (HAECs) | Mouse AS model | Increased the endometrium and the number of endometrial glands, reduced fibrosis, generated microvessels. | Rich sources; convenient obtaining condition; no ethical issues; low immunogenicity; no tumorigenicity | Little basic researches. | [39] |
2.1. Endometrial stem cells
The human endometrium is a complex and dynamic tissue that experiences a period of growth and shedding during each menstrual cycle, suggesting the existence of stem cells [40]. The endometrium consists of two regions: basal layer and functional layer. Endometrial stem cells are located in the basal layer and are the source of progenitor cells that differentiate to form the endometrium. These endogenous progenitor stem cells can rapidly replace the functional layer of the endometrium during each menstrual cycle and have the potential for high proliferation, self-renewal, and multilineage differentiation [41]. Endometrial stem cells have been shown to differentiate into various cell types such as adipocytes, chondrocytes, muscle cells, cardiomyocytes and neurons [42]. Exogenous endometrial stem cells can differentiate into endometrial cells in the uterus, thus a potential therapy for uterine injury [[42], [43], [44], [45], [46], [47]]. Endometrial stem cells have been shown to be ideal seed cells for the treatment of infertility caused by endometrial damage [48]. However, obtaining endometrial stem cells directly from the endometrium may cause great damage. One study show that exfoliated endometrial cells isolated from menstrual blood can be cultured into endometrial mesenchymal stem cells in vitro [49]. The cells have higher proliferative capacity and can be stably differentiated for more than 30 generations in vitro, which can be directly used to promote the repair of endometrial damage in nude mice [50]. In addition, menstrual blood stem cells (MenSCs) can improve the implantation rate of embryos, which may be related to their promotion of the expression of vimentin, keratin and vascular endothelial growth factor [51].
2.2. Bone marrow stem cells
Bone marrow-derived cells (BMDCs) are the earliest and most widely studied mesenchymal stem cells. BMDCs can transdifferentiate into a variety of non-hematopoietic cell lines, including skin, muscle cells, neurons, hepatocytes, cardiomyocytes, and gastrointestinal epithelial cells [[52], [53], [54]]. In addition, BMDCs can also differentiate into cells related to endoderm, mesoderm, and ectoderm, including various mature endometrial cells [[55], [56], [57], [58], [59]]. Through bone marrow transplantation, bone marrow mesenchymal stem cells can migrate to the injured area, and then re-proliferate and differentiate into various organs in the host [15,[60], [61], [62], [63]], that is, participate in tissue regeneration. There are many reports on the use of bone marrow mesenchymal stem cells in the treatment of endometrial injury, and positive results have been obtained in rodents and clinical patient experiments [[30], [31], [32]]. BMDCs can differentiate into epithelial cells and stromal cells in the recipient's endometrium and vascular endothelial cells [43,57], playing multiple roles in the repair of endometrial damage such as promoting endometrial reconstruction [55,[64], [65], [66], [67]]. However, its application is still facing great challenges, mainly due to the great pain and concomitant damage caused by cell collection to the donors, as well as the detrimental effects on the psychology of both the donor and the recipient [68]. In addition, there are huge individual differences in the proliferation, survival, differentiation and paracrine capacity of MSCs in vitro, which makes it difficult to unify and standardize the research results as guidelines for clinical treatment, limiting their application in clinical treatment of IUA [[69], [70], [71]].
2.3. Cord blood stem cells
Human umbilical cord-derived mesenchymal stem cells (hUMSCs) are another rich source of mesenchymal stem cells. Due to its high safety, low immunogenicity, high proliferative capacity and easy collection, it has become another effective candidate for tissue regeneration after endometrial injury [64,72]. Studies have shown that hUMSCs can promote vascular remodeling, immune cell recruitment, and produce a large number of beneficial active molecules, thus playing a key role in repairing endometrial damage [73]. Other studies have shown that exosomes of umbilical cord mesenchymal stem cells played an active role in the repair of damaged endometrium through the PTEN/Akt signaling pathway [74].
2.4. Adipose stem cells
Adipose tissue is relatively abundant in the human body, and adipose stem cells (ASCs) can be easily isolated from subcutaneous adipose tissue such as thighs, abdomen, and arms [75,76]. Studies have shown that the number of stem cells obtained from adipose tissue is much larger than that obtained from an equivalent amount of bone marrow [77]. ASCs with pluripotent differentiation have also been shown to have great potential in clinical application [[78], [79], [80]]. In fact, ASCs have higher proliferative capacity and anti-aging ability than BMSCs. In addition, ADSCs have greater secretion capacity than MSCs and secrete interleukin-6 (IL-6) and insulin-like growth factor-1 (IGF-1) which can further enhance the therapeutic and regenerative effects of stem cells [81], thus having great significance for cell therapy and tissue regeneration [82]. Currently, clinical therapies using ADSCs have achieved good results, including wound healing, skin regeneration, and tissue regeneration [75,76]. Therefore, ADSCs may also be used to treat IUA in the future.
3. Mechanism of stem cells to repair endometrial damage
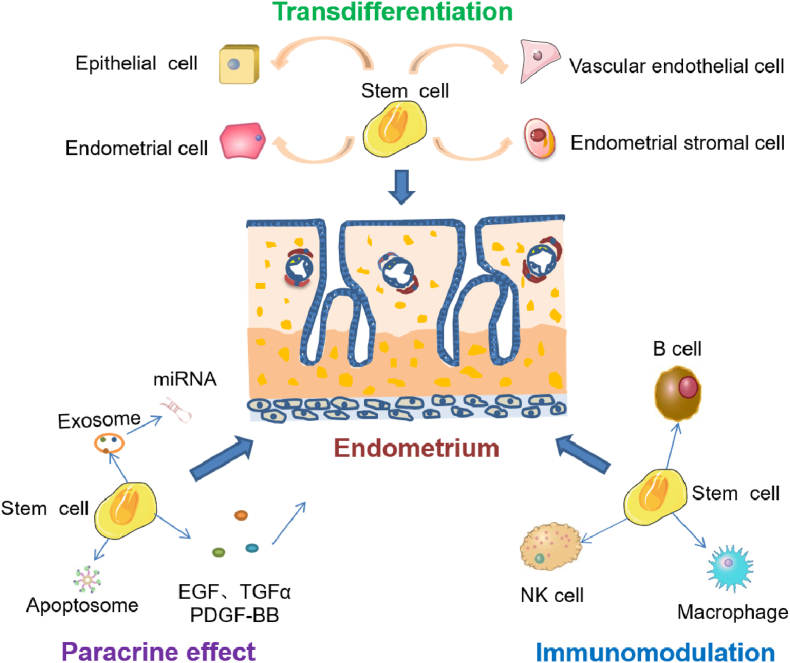
Stem cells repair endometrial injury mainly due to their differentiation ability, paracrine activity, and immunomodulatory effects (Fig. 3).
Fig. 3.
Mechanism of stem cells to repair endometrial damage.
3.1. Direct differentiation of stem cells into functional cells
It has been reported that transplantation of mesenchymal stem cells (MSCs) or endometrial stem cells into the uterine cavity of IUA patients or female rats can promote endometrial regeneration, which might be due to the potential differentiation ability of stem cells [83]. In addition to direct differentiation into endometrial cells, pluripotent stem cells (PSCs) can also differentiate into other cells such as stromal cells and vascular endothelial cells, which are involved in endometrial repair [84]. Moreover, in addition to differentiating into functional cells, PSCs can also regulate the behavior of environmental B cells, NK cells, and macrophages by secreting cytokines and chemokines, thus playing a role in repairing the endometrium to Refs. [85,86].
3.2. Paracrine activity of stem cells
Discovered in the 1940s, exosomes are membrane vesicles with a diameter of 30–150 nm, containing proteins, lipids, nucleic acids and other key substances that regulate biological functions, and their biological functions vary according to the cells that secrete them [3]. Exosomes can act as messengers for intercellular communication and alter the fate of cells by regulating target genes [[4], [5], [6],11]. Recent studies have shown that exosomes derived from stem cells, as paracrine factors, can regulate the expression of related genes in recipient cells to achieve cell and tissue regeneration [10,13,14,20,87].
At present, a number of studies have proved the vital role of exosomes derived from various stem cells, including bone marrow-derived mesenchymal stem cells, umbilical cord blood mesenchymal stem cells, menstrual blood-derived endometrial stem cells, and adipose-derived mesenchymal stem cells, in the repair of endometrial injury. They act by changing signaling pathways, delivering miRNAs, releasing cytokines, or altering functional proteins [[88], [89], [90]]. For example, exosomes derived from human umbilical cord mesenchymal stem cells can promote the proliferation of human endometrial stromal cells, protect human endometrial stromal cells from mifepristone-induced apoptosis, and repair damaged endometrial stromal cells through PTEN/Akt signaling pathway [2]. Through the identification of the differential expression profile of miRNA in human umbilical cord mesenchymal stem cells and endometrial epithelial cells using miRNA microarray, it was found that exosomes from human umbilical cord mesenchymal stem cells contained miR-7162–3p, which could reduce the mifepristone-induced apoptosis of endometrial epithelial cells by regulating APOL6 [8]. Exosomes produced by BMSCs also regulate the repair of damaged endometrium by effectively transferring miR-340 to endometrial stromal cells, or by the TGF-β1/Smad signaling pathway [17]. Moreover, together with BMSCs, they can significantly increase the expression of CK19 and reduce the expression of VIM, TGF-β1, TGF-β1R and Smad2, thereby tremendously increasing the number of glands in the damaged endometrial tissue and reducing the area of fibrosis, leading to the recovery of the function of the damaged endometrium in rats [18]. Studies have shown that UC-MSCs-derived exosomes on collagen scaffolds could be used for endometrial regeneration in a rat model of endometrial injury by macrophage immunomodulation, and facilitate the polarization of CD163+ M2 macrophages in vivo and in vitro through the immunomodulation of miRNA, thereby reducing inflammation [91]. Moreover, human umbilical cord mesenchymal stem cell-derived exosomes can significantly inhibit the release of IL-6 and IL-1β and the expression of TLR4 and RelA, and promote the release of TNFα in damaged endometrial epithelial cells, thereby significantly reducing the cell viability and inhibiting the release of lactate dehydrogenation in a concentration-dependent manner [12]. In addition, these exosomes are also involved in the regulation of the immune system, thereby regulating the inflammatory response of damaged tissues to repair the endometrium [7,[92], [93], [94]]. Adipose mesenchymal stem cell-derived exosomes promote endometrial regeneration and collagen remodeling by enhancing the expression of integrin-β3, LIF and VEGF, thereby restoring fertility in a rat model of IUA [9].
In addition to the above exosomes, a cell-free therapeutic strategy was achieved by mesenchymal stem cell-derived apoptotic bodies (Abs) to obtain endometrial regeneration and fertility restoration, in which Abs can induce the immunomodulation of macrophages, cell proliferation, and angiogenesis in vitro [95]. Another study reported that mesenchymal stem cell-derived secretions (MSC-Sec) restored injured endometrial morphology and fertility in a rat model [96]. The extracellular vesicles (EVs) of MSCs contain microRNA let-7a-5p, which helps reduce apoptosis and enhance autophagy in kidney repair [97]. Therefore, with the in-depth study of such secretions with membrane structures, cell-based therapeutic research has gradually shifted to non-cellular active substances [98]. Studies have shown that mesenchymal stem cell-derived exosomes are safe and effective, and especially have significant advantages in reducing the tumorigenicity of cell administration [60,61]. Mesenchymal stem cell-derived exosomes have emerged as a new cell-free therapeutic strategy as a substitute for mesenchymal stem cells in various disease models, including neurological, cardiovascular, immune, renal, musculoskeletal, liver, respiratory, eye and skin diseases, as well as cancers [15,62,63,99,100].
Moreover, growth factors produced by stem cells can also exert paracrine activity, that may have synergistic and regulatory effects on the behavior of stem cells. During the repair and regeneration of tissues, colony-forming cell units (CFUs) need the regulation of growth factors, including epidermal growth factor (EGF), transforming growth factor (TGF), and platelet growth factor BB (PDGF-BB). PDGF-BB has an important role in endometrial tissue remodeling [46]; EGF determines the survival, proliferation, differentiation and migration of cells through interaction with EGF receptors, which is essential for organ repair and wound healing [47]; whereas upregulation of TGF-β is closely associated with the development of fibrotic diseases [101], e.g., endogenous TGF-β is essential for hypertrophic remodeling and pathogenesis of cardiac fiber remodeling [102]. Studies have found that the levels of EGF, PDGF-BB, and TGF-β were significantly increased in IUA rats compared with the control group [101], so it is speculated that the above growth factors may be involved in the development of IUA [19]. Bone marrow-derived stem cells can directly secrete a variety of cell growth factors such as hepatocyte growth factor, platelet-derived growth factor, and transforming growth factor-β, which in turn have effects on the cells and tissues of the recipient [42]. Studies have also shown that bone marrow derived-mesenchymal stem cells improved regeneration of fresh wounds of the uterine wall by secreting fibroblast growth factor 2 (FGF2), insulin-like growth factor 1 (IGF1) and vascular endothelial growth factor (VEGF) [2]. Basic fibroblast growth factor (bFGF) can also promote healing of refractory ulcer and contributes to angiogenesis of tissues [16]. Therefore, the damaged endometrium can be effectively repaired by direct delivery of growth factors through scaffolds or the addition of growth factors in addition to stem cells. For example, menstrual blood-derived endometrial stem cells work together with platelet-derived growth factor (PDFG) to synergistically increase the expression of CD34 in damaged endometrial tissue and significantly enhance the phosphorylation of Akt and Bad in endometrial tissue, thereby promoting the repair of endometrial injury [1].
3.3. Other mechanisms of stem cells promoting tissue repair
Research on the mechanism by which stem cells promote tissue repair is still ongoing. So far, some new mechanisms and targets have been discovered, such as ΔNp63, MMP-9 and immune regulation. Loading collagen scaffolds containing bone marrow mononuclear cells (BMNCs) on the endometrium of AS patients can downregulate the expression of ΔNp63, reverse ΔNp63-induced pathological changes, normalize the stemness alterations, and restore endometrial regeneration [31]. Umbilical cord-derived mesenchymal stem cells loaded into scaffolds facilitate collagen degradation by upregulating MMP-9 in rat uterine scars, which is positively correlated with the pregnancy rate [2]. More interestingly, the ability of the recipient to generate apoptotic MSCs has been proved to be the essential mechanism which explains the paradox that MSCs still have therapeutic effects despite the lack of engraftment [103]. As research continues, the interconnections and signal networks between these targets will become increasingly clear.
4. Clinical application of stem cells and improvement strategies
Various methods have emerged for stem cell transplantation. For instance, intrauterine transplantation [31] and tail vein injection [104,105] have been used in the preclinical studies of MSCs for the treatment of IUA. In limited clinical studies using stem cells to treat IUA, the methods used include local intrauterine injection [106], subendometrial injection [32], intrauterine spiral artery injection [28], etc. The effect of intravenous injection of stem cells on endometrial regeneration has been reported to be limited [104]. Therefore, many scientific studies have been conducted on how to improve the implantation effect and long-term effectiveness of grafts in clinic, avoid tumor formation, and facilitate storage and transportation. In terms of the pathological features and treatment requirements of IUA, functional bioscaffolds or multifunctional platforms are very important for the application of stem cells.
4.1. In situ uterine stents
Due to their unique 3D structures, in situ uterine stents can separate the injured endometrium and avoid adhesion reforming. In addition, they can be used as vehicles to deliver stem cells in various therapies, providing a better solution for the treatment of IUA [107]. Among them, estrogen hydrogel has been used clinically to prevent postoperative IUAs [108]. Collagen scaffolds which carried umbilical cord MSCs have been at phase I clinical trial for patients with recurrent uterine adhesion [33]. Essentially, smart polymeric nano-systems with appropriate composition can be defined as artificial scaffolds to mimic the morphology, structure, and function of the surrounding tissue. Meanwhile, scaffolds should also be capable of enhancing the function of cells, such as cell adhesion, differentiation, maintenance, and migration, and the autocrine of growth factors, immunomodulators, and other bioactive factors. Therefore, when selecting scaffold materials, their interactions widths stem cells should be considered from the aspects of connectivity, pore size and shape. The appropriate morphology of biomaterial scaffolds, especially their microstructures, is important for cell differentiation and tissue response [109].
Hydrogels have been widely used for anti-IUA barrier materials because of their ability to form mechanical intrauterine cavities to reduce the formation of fibrous tissue [103]. The uterine cavity is surrounded by a muscular layer of interlacing smooth muscle fibers, which usually causes the uterine cavity to collapse during the postoperative healing process unless it is mechanically distended [110]. Therefore, the application of hydrogels needs to meet the following requirements: First, the size and shape of hydrogels can be adjusted to suit different uterine cavities. Second, it is easily degraded or absorbed naturally and remains in the uterine cavity long enough for treatment [111]. Moreover, temperature-responsive hydrogels exhibited better performance, responding rapidly to physical and chemical changes for the attachment of stem cells and preventing their rapid outflow from uterine cavity. According to the characteristics of the materials used, the major scaffold systems are discussed in detail as follows.
4.1.1. Polymer-based hydrogels
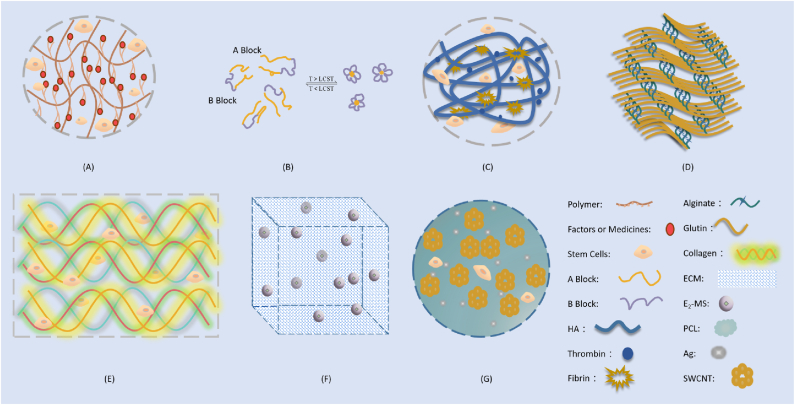
Synthetic polymer scaffolds have shown great potential in tissue engineering as they can be tailormade with controllable architectures to present diverse functionalities and be standardized for mass production [112]. Many kinds of polymer-based hydrogels have been studied and even used for IUA treatment (Fig. 4) [111]. Pluronic F-127, a Food and Drug Administration (FDA)-approved synthetic polymer with the advantages of low toxicity, biocompatibility, and thermal reversibility, can form hydrogels at physiological temperatures [113]. A Pluronic F-127 hydrogel carrying bone marrow stromal cells (BMSCs) and vitamin C was prepared to promote restoration of IUA-damaged endometrium in vivo [114]. A thermosensitive aloe-poloxamer (AP) hydrogel was applied to embed β-estradiol (E2)-encapsulated nanoparticulate decellularized uterus (uECMNPs) E2. This multiple components of the prepared E2@uECMNPs/AP system could collectively promote endometrial regeneration and prevent re-adhesion [115]. A temperature-sensitive heparin-modified poloxamer (HP) hydrogel was reported with affinity to keratinocyte growth factor (KGF), a potent repair factor for epithelial tissues. The resulting KGF-HP was used as a support matrix to prevent IUA [116].
Fig. 4.
Major scaffold systems for IUA treatment in stem cell therapy. A: Polymer-based hydrogel, polymer could be Pluronic F-127, AP, HP, PLGA, PGS, PEG, GelMA or ColMA. Polymer-based hydrogels can encapsulate multiple components like stem cells, drug molecules, growth factor, etc [18,19,[114], [115], [116], [117], [118], [119], [120], [121]]. B: Polymer-based hydrogel, polymer could be ABA-type triblock copolymers (A Block: DEAEMA-co-MEO2MA-co-OEGMA, B Block: PEG [119]); LCST, low critical solution temperature. C: Polysaccharide-based hydrogels, e.g. Fibrin/HA-Stem Cells [124]; D: Protein-based hydrogels, e.g. 3D-printed gelatin/alginate hydrogel [136]; E: Protein-based hydrogel, e.g. Collagen hydrogel [134]; F: Cellular component materials: e.g. ECM scaffold [148]; G: Other materials, e.g. single walled carbon nanotubes (SWCNTs) [149].
Poly (glycerol sebacate) (PGS), another representative of synthetic bioelastomers, has been widely used in a variety of biomedical fields, especially in soft tissue regeneration. PGS porous scaffolds have shown to be good carriers for numerous types of cells such as human umbilical vein endothelial cells, mesenchymal stem cells, and bone marrow stromal cells [18,19]. A scaffold made of elastic poly PGS and poly (lactic-co-glycolic acid) (PLGA) was utilized to load BMSCs in resumption of damaged rat uteruses, the 3D architecture of which was beneficial to the adhesion and growth of rat BMSCs [117]. Injectable poly (ethylene glycol)-b-poly (l-phenylalanine) hydrogels showed the potential in the prevention of uterine adhesion owing to its effect on preventing fibrosis after uterine tissue injury and promoting pregnancy [118]. Moreover, an ABA-type triblock copolymer, p (DEAEMA-co-MEO2MA-co-OEGMA)-b-PEG-b-p (DEAEMA-co-MEO2MA-co-OEGMA), composed of a poly (ethylene glycol) (PEG) middle block and temperature- and pH-sensitive outer blocks, was synthesized by atom transfer radical polymerization (ATRP). The triblock copolymer, which contains a small amount of a weak base group, (N, N-diethylamino) ethyl methacrylate (DEAEMA), showed lower critical solution temperature (LCST) and could be tuned precisely and reversibly by changing the pH of the solution. When the copolymer concentration was high enough, an increase in temperature transformed the free-flowing solution into a micellar gel. The sol-to-gel transition temperature (Tsol–gel) in aqueous solution decreased with increasing concentration [119].
Another new bioactive hydrogel scaffold, gelatin methacryloyl (GelMA), can be used as a sustained-release drug delivery system to effectively promote endometrial repair in IUA animal models. By adjusting the concentration of GelMA, the hydrogel substrates with the variation of stiffnesses influenced neuronal growth, including cell viability, adhesion, spreading, and average neurite length [120]. Tissue hydrogels with various proportions of GelMA and methacrylated collagen (ColMA), loaded with amniotic mesenchymal stem cells (AMSCs), were constructed using 3D biological printing technology to prevent cavity adhesion in an IUA rat model. The carried cells could be released continuously for more than 7 days with the function of normal cells [121].
4.1.2. Polysaccharide-based hydrogels
Self-crosslinked hyaluronic acid gel (HA-GEL), a promising physical barrier composed of a natural mixture of extracellular matrix and synovial fluid, has been approved by China Food & Drug Administration (CFDA) as a medical device for clinical practice. The effectiveness of HA-GEL in preventing IUAs was reported by American Association of Gynecologic Laparoscopists in 2017 [122]. It was used in the treatment of uterine cavity because of its advantages of prolonged absorption time (7–14 days), good expansion, anti-inflammatory, and repairing effect. It was shown that HA-GEL loaded with human umbilical cord–derived mesenchymal stem cells facilitated intrauterine reconstruction and endometrial regeneration in an IUA model [123]. In order to optimize the short half-life of natural HA, endometrium-tailored HA/fibrin composite hydrogel HA-fibrin-thrombin 50 mg (HA-F-T50) was designed [124]. Kim et al. found that in the mouse model of intrauterine infertility, decidua endometrial stromal cells (dEMSC) and fibrin/HA hydrogel could synergistically increase the intimal regeneration, and ultimately promote pregnancy, because fibrin/HA hydrogel could maintain the decidualisation characteristics of dEMSC, which was reflected in the high expression of IGF-I in the fibrin/HA-dEMSC group, while the biocompatibility and adhesion of fibrin/HA hydrogels were also improved. In addition, thrombin may be an auxiliary molecule that enhances the viscosity and biocompatibility of therapeutic delivery systems [124]. Moreover, polyurethane (PU)/starch (St) HA (co-axial) was found to increase cell adhesion and facilitate skin wound healing in male rats [125], suggesting the widespread use of HA hydrogels.
Among 3D bioactive hydrogel scaffolds, chitosan is another biopolymer with unique biological and physicochemical properties. Derived from chitin, chitosan is a natural, biocompatible and biodegradable polysaccharide material with a structure similar to that of glycosaminoglycan [126]. As a major component of extracellular matrix (ECM), chitosan exhibited antibacterial activity, good cell adhesion and proliferation properties [127]. It has been found that gel/chitosan/bioglass nanofiber scaffolds (BGNPs) played an important role in angiogenesis and endothelial cell differentiation. Based on their large surface area, composition, and ability to enhance cell viability, GEL/CS/BGNPs nanofibers have been proved to be favorable scaffolds for angiogenesis and tissue regeneration and healing. Therefore, GEL/CS/BGNPs nanofiber scaffolds can be used for the proliferation and differentiation of endothelial stem cells, which is a promising scaffold for the treatment of endometrial damage [128]. Zhang et al. prepared a thermo-responsive hydrogel containing galactose-modified xyloglucan (mXG) and hydroxybutyl chitosan (HBC) as an injectable anti-adhesion material with spontaneous gelling behavior at the body temperature for clinical treatment of IUAs [129].
4.1.3. Protein-based hydrogels
Protein-based biomaterials offers unique opportunities to engineer biofunctionality, biocompatibility, and biodegradability with the exquisite control of their composition, stereochemistry, and chain length. Collagen, one of the basic structural elements of the extracellular matrix, is widely used in wound repair and tissue regeneration due to its abundance, biodegradability and biocompatibility [[130], [131], [132], [133]]. Collagen is not only a framework that supports tissue structure, but also a substance that regulates cell behavior, including cell adhesion, migration, and differentiation. Transplanting clinical-grade umbilical cord-derived mesenchymal stromal cells into a degradable collagen scaffold is a safe and effective clinical therapy for the treatment of postoperative recurrence of IUA after adhesiolysis surgery [33]. Ding et al. found that bone marrow mesenchymal stem cell-encapsulated collagen scaffolds could improve the repair of severely damaged endometrium in a rat model [134]. In addition, gelatin is a biocompatible and biodegradable protein with a chemical composition very similar to that of native collagen, and thus can serve as a suitable candidate material in many scientific fields, such as biomimetic scaffolds in tissue engineering, which has the potential to achieve tissue manipulation, regeneration, and growth [135,136]. Currently, as a personalized and precise treatment strategy, “cellular 3D printing” is more in line with the clinical requirements of tissue repair. For example, 3D-printed gelatin/alginate hydrogel scaffold loaded with human induced pluripotent stem cell-derived mesenchymal stem cell (hiMSC) restored embryo implantation and pregnancy maintenance in some patients with damaged endometrium [136]. In addition, protein-based polymers such as elastin-like polypeptide (ELP) combined with periodic cysteine residues (cELPs) were thermally responsive and could form gels rapidly under mild oxidative conditions [137].
4.1.4. Cellular component complex materials
Biomaterials, which can properly simulate the physicochemical properties of the intracellular or extracellular environment, are considered a key component of tissue engineering [138,139]. They can help restore the structural and functional properties of damaged tissue by providing a three-dimensional microenvironment [140]. In order to treat endometrial injury, some researchers have attempted to construct a 3D artificial endometrium rich in endometrial stem cells by simulating the complex physiological and structural properties of the human endometrium [141]. A variety of biomaterials have been reported to mimic the multicellular and multilayered structure of the human endometrium by incorporating various endometrial cellular components (derived from vascular endothelial cells, stromal cells, and endometrial stem cells) [142]. Among them, collagen and hyaluronic acid, as the main components of the ECM, have been successfully used in many fields of tissue engineering and regenerative medicine applications due to their relative abundance, inherent biodegradability, and excellent biocompatibility [139,[143], [144], [145]]. An example is decellularized amniotic membrane (AM), which is a promising material in human tissue repair and regeneration due to its biocompatibility, biodegradability, and preservation of abundant bioactive components [146]. A drug delivery system based on human amniotic extracellular matrix (HAECM) was developed to facilitate endometrial regeneration [147]. Another example is that well dispersed 17β-estradiol (E2)-loaded PLGA microspheres (E2-MS) in the scaffolds, realizing the sustained release of E2 for 21 days [148]. In addition, UC-MSCs/Matrigel microspheres with controllable particle size and cell encapsulation capacity showed a great effect on the repair and regeneration of the endometrium [14].
4.1.5. Other materials
The electrical properties of biomaterials can be modulated by introducing conductive nanomaterials such as carbon nanostructures (e.g., nanotubes, graphene, and nanofibers) and metal nanostructures (e.g., gold, silver). Studies have shown that the integration of two different nanostructures helps improve the performance of biofunctional porous scaffolds, ranging from specific bioactivity, structural and mechanical integrity, to electrical conductivity. One example is the combination of single walled carbon nanotubes (SWCNTs) and silver nanoparticles (Ag) with a biodegradable polymer matrix. Different amounts of SWCNT were mixed with Ag nanoparticles and introduced into a poly (e-caprolactone) (PCL) polymer matrix by solvent casting method to obtain PCL/Ag/SWCNTs which showed good biocompatibility with human bone marrow mesenchymal stem cells (hBM-MSCs), thereby providing a new perspective for their biomedical applications [149]. Some biomaterials such as polymer-based hydrogel, polysaccharide-based hydrogels, protein-based hydrogels, and cellular component materials are summarized in Fig. 4.
4.2. Multifunctional platforms for stem cell therapy
From the progress of the above scaffold materials, in addition to realizing the functions of physical barrier and loading stem cells, the development of scaffolds gradually shows the characteristics of multi-function, multi-target and multi-mechanism, such as controlled release of drugs, collaborative repair, and comprehensive treatment. The main functional components of the scaffolds are as follows.
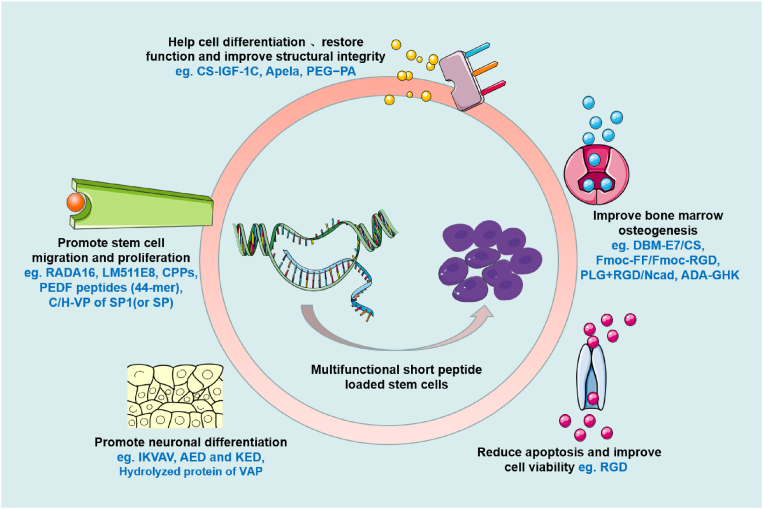
4.2.1. Multifunctional short peptides
There is considerable evidence that biomaterial scaffolds can mitigate harmful injury and create a nurturing and protective environment, thereby enhancing the therapeutic effect of implanted cells. Engineered materials are ideal scaffolds to provide biomimetic 3D systems to support the interaction of nanobiomaterials and stem cells, and guide the behavior of stem cells. However, the clinical application of donor cells is greatly limited due to their low survival rate, decreased proliferative activity, weak homing targeting effect to damaged area, and low adhesion ability after transplantation [88]. Co-transplantation of stem cells with functional factors can improve cell engraftment, but this strategy has been hampered by the short half-lives of the factors and the fact that the scaffolds have not been chemically determined [150]. Thus, the introduction of multi-functional short-lived peptides provides better support for regulating the behavior of stem cells after transplantation (Fig. 5). For example, a simple collagen-dendrimer biomaterial was crosslinked with pro-survival peptide analogues attached to the extracellular matrix to achieve slow release of the peptides, significantly prolonging the survival of stem cells in a mouse model of ischemic injury [151]. Moreover, laminin isoforms, laminin-511 and its E8 fragment (LM511E8), which are the major components of basement membranes, have been shown to strongly promote the adhesion and proliferation of human pluripotent stem cells. A collagen scaffold with the adhesion activity of laminin-511 cells was fabricated and showed a proliferative effect on human iPS cells [152]. After the synthetic IGF-1C peptide and adipose-derived mesenchymal stem cells (ADSCs) were introduced into chitosan hydrogels (CS-IGF-1C), it was found that the functional recovery and structural integrity of injured organs were significantly improved [153]. Moreover, a composite scaffold combining mesenchymal stem cells (MSCs) E7 affinity peptide-modified demineralized bone matrix (DBM) particles and chitosan (CS) hydrogel was constructed for cartilage engineering. DBM-E7/CS scaffold significantly promoted the survival of rat bone marrow-derived MSCs (BMMSCs) by increasing matrix production and improving chondrogenic differentiation ability of BMMSCs [154]. Another biofunctionalized scaffold relied on arginine-glycine-aspartic acid (RGD), a self-assembling peptide with a specific cell recognition motif that binds strongly to the integrin of stem cells, triggering integrin-stimulated cell adhesion. RGD hydrogels were developed to enhance the efficacy of MSC-extracellular vesicles (EV) in a model of acute kidney injury [97]. In addition, the combination of multi-segment functional peptides presents more advantages: A class of functionalized self-assembling peptide nanofiber scaffolds were developed from self-assembling peptide RADA16-I (AcN-RADARADARADARADA-CONH2). Short peptide motifs SKPPGTSS (bone marrow homing motif), FHRRIKA (heparin-binding motif) and PRGDSGYRGDS (two-unit RGD cell adhesion motif) were used to extend the C-terminus of RADA16-I to obtain functionalized peptides. Human adipose stem cell (hASCs) cultured in this 3D peptide hydrogel exhibited enhanced behaviors including migration, proliferation, and the ability of secreting growth factors [155].
Fig. 5.
Common multifunctional short peptides and their positive effects on stem cell transplantation.
4.2.2. Controlled release of functional molecules
Some active substances that contribute to wound healing and angiogenesis or prevent fibrosis or regulate stem cell behavior have also been selected to be embedded in scaffolds for IUA treatment, mainly including chemokines, growth factors and other active functional protein molecules (see Table 2). Studies have shown that cytokines and chemokines secreted by damaged tissues are the key to homing of MSCs to the site of injury [99]. Stromal cell-derived factor 1, expressed on various MSCs, is a ligand for the receptor, chemokine receptor 4, which has been shown to play a key role in the homing process [100]. In addition, irradiation, chemotherapy, or hypoxia can promote the expression and secretion of stromal cell-derived factor 1, resulting in the local recruitment of MSCs to damaged tissues [156]. Encapsulation of these factors into scaffolds is conducive to the synergistic effect of tissue repair and stem cell therapy. For example, chitosan-heparin hydrogels were used for the sustained release of SDF-1α to treat intrauterine adhesion. Endogenous c-kit positive stem cells (HSCs) were recruited to the site of injury to promote the healing of intrauterine adhesion [157]. Keratinocyte growth factor (KGF), a potent repair factor for epithelial tissues, was loaded into a temperature-sensitive heparin-modified poloxamer (HP) hydrogel to form a therapy (KGF-HP) for the treatment of IUA [116].
Table 2.
Multifunctional platforms loaded with active functional factors on endometrial diseases.
| Factors | Scaffold materials | Disease model | Mechanism | References |
|---|---|---|---|---|
| Stromal cell-derived factor-1 (SDF-1) | Chitosan-heparin hydrogels | Rat intrauterine adhesion; uterine injury | Controlled release of SDF-1α increased endogenous c-kit positive stem cells (HSCs) recruited to the injury site with enhanced endometrial regeneration and arteriogenesis of the injured rat uterus, which led to improved pregnancy outcomes. | [157,158] |
| bFGF | A collagen membrane loaded with bFGF fused a collagen-binding domain (CBD) to the N-terminal which limits the diffusion of bFGF from collagen. | Rats under the severe uterine damage model | Promoting the proliferation and differentiation of stromal cells, facilitating the formation of blood vessels; improving regeneration abilities of uterine endometrium and muscular cells, achieving better pregnancy outcomes in rats. | [159] |
| bFGF | Collagen bound to bFGF around fibrosis | A pilot study of human uterine injury | Promoting the proliferation and differentiation of stromal cells and the formation of blood vessels | [16] |
| bFGF | A microfluidic droplet template which combines the characteristics of the artificial biocompatible material GelMA and the natural polysaccharide material Na-alginate | Rat model of IUAs | Improving neovascularization, cellularizing the damaged tissue, and repairing the endometrium. | [160] |
| Keratinocyte growth factor (KGF) | A temperature-sensitive heparin-modified poloxamer (HP) hydrogel | Rat injured uterus model | Increasing proliferation of endometrial glandular epithelial cells and luminal epithelial cells; facilitating angiogenesis of injured uterus. | [116] |
| KGF | Using heparin-modified poloxamer (HP) as the matrix material and ε-polylysine (EPL) as functional excipient | Human endometrial injury | Enhancing the proliferation of endometrial epithelial cell and glands, as well as angiogenesis in the regenerated endometrium. | [161] |
| Vascular endothelial growth factor (VEGF) | a collagen-binding VEGF by fusing a collagen-binding domain to the N-terminal of native VEGF | Rat scarred uterus model | Promoting remodeling of the scarred uterus including the regeneration of endometrium, muscular cells, and vascularization and improved pregnancy outcomes. | [162] |
4.2.3. Different functional drugs
For the treatment of endometrial injury, in addition to stem cell therapy, it has been found that it has a good synergistic effect with drugs with various functions such as antibacterial, anti-inflammatory, promoting angiogenesis, promoting cell proliferation, promoting stem cell homing, and promoting damage repair, so as to achieve better therapeutic effects.
Among them, estrogen is one of the most important female sex hormones, which plays a key role in the regulation and development of the female reproductive system and the formation of secondary sexual characteristics. For example, postoperative estrogen therapy can be used to prevent adhesion recurrence [163] and promote endometrial regeneration [[52], [53], [54]]. In addition, estrogen promotes the levels of ESR1, MMP-9, EGF and IGF-1 by inhibiting the increase in the levels of serum TGF-β1, epidermal growth factor, and PDGF-BB mediated by IUA- [64]. Therefore, these advantages provide a new option for the treatments of IUA with drug-loaded bioactive scaffolds. Studies found that compared with embryonic stem cell transplantation alone, its combination with estrogen therapy can improve the therapeutic effect [164]. For instance, 17β-estradiol (E2) can be sustainably released for 21 days by a human amniotic extracellular matrix (HAECM) scaffold integrated with E2-loaded PLGA microspheres [148]. The sustained release of E2 was achieved by encapsulating E2 in heparin-poloxamer (HP) micelles to construct a temperature-sensitive hydrogel. Prolonged estrogen release in the target area of IUA rats, accompanied by increased levels of VEGF, PI3K/Akt, and ERK1/2, significantly inhibited the endoplasmic reticulum stress [165]. Antibiotics, proliferative drugs, antidiabetic drugs, drugs that can increase endometrial blood flow are listed in Table 3.
Table 3.
Multifunctional platforms loaded with chemical drugs.
| Loaded drug | Mechanism | Diseases and Curative Effects | References |
|---|---|---|---|
| 17β-estradiol | Estrogen. Significantly inhibits IUA-increased TGF-β1, epidermal growth factor and PDGF-BB levels; promotes ESR1 levels; promotes PI3K/Akt and ERK1/2 signaling activation, and inhibits endoplasmic reticulum stress. | Postoperative estrogen therapy can be used to prevent recurrent adhesions; estrogen can increase the number of cells in endometrial damage and promote endometrial regeneration; inhibit endoplasmic reticulum stress-related apoptosis | [145] |
| Sitagliptin | Dipeptidyl peptidase IV (DPP4) inhibitor. Promotes stem cell homing and enrichment to the site of tissue damage | Inhibits the expression of DIO2, a marker gene of senescent decidual cells, increases endometrial-media embryonic stem cells, and reduces decidual senescence | [166] |
| Pentoxyphene | Medications that increase endometrial blood flow | Treats endometrial damage | [167] |
| Tocopherol | |||
| Sildenafil | |||
| Vc | Regulatory factor. Promotes stem cell survival, promotes endometrial recovery, promotes keratin, vWF expression recovery, reduces IL-1β | Attenuates the cytotoxic effect of PF-127, promotes cell survival and growth during encapsulation of rat bone marrow mesenchymal stem cells, and promotes intimal regeneration. | [114] |
| Mitomycin C | Antibiotics. | Inhibits the cell viability of endometrial stromal cells, promotes G1 cell cycle arrest and apoptosis, and inhibits the synthesis and secretion of type I collagen. | [168] |
| Metformin | Anti-diabetic drugs. | Inhibits ER stress-induced apoptosis through PI3K/Akt and ERK1/2 pathways. | [169] |
| Silver ions | Fungicide. Exhibits anti-infective effect. | Works synergistically with other ingredients facilitating endometrial regeneration, fertility restoration, and live birth of offspring | [170] |
4.2.4. Other complex active ingredients
With the deepening of research, it has been found that active components such as exosomes, microvesicles, secretome, and apoptotic bodies are crucial to the function of stem cells. In addition, some other biological components with synergistic repair activities such as platelet-rich plasma have also been attempted for the construction of multifunctional platforms. Relevant studies are summarized in the table below (Table 4).
Table 4.
Multifunctional platforms loaded with active bodies with membrane structures.
| Active Ingredient | Role | Mechanism | Loading Platform | Reference |
|---|---|---|---|---|
| Adipose stem cell-derived exosomes (ADSC-exo) | Promote endometrial regeneration and fertility recovery | Reduce infection risk, promote neovascularization and tissue regeneration, while inhibiting local tissue fibrosis | PEG hydrogel | [170] |
| Platelet-rich plasma (PRP) | Promotes proliferation and migration of chondrocytes and bone marrow stem cells (BMSCs) | Controlled release of growth factors to enhance tissue adhesion, facilitate cartilage defect regeneration. Our further in vitro experiment showed that HNPRP hydrogel could promote the proliferation and migration of chondrocytes and bone marrow stem cells (BMSCs) | photoresponsive hyaluronic acid | [171] |
| Mesenchymal stem cell-derived exosomes | Promote endometrial regeneration | Promote CD163+ M2 macrophage polarization, reduce inflammation, and increase anti-inflammatory responses in vivo and in vitro through immunomodulatory functions of miRNAs | Collagen scaffold (CS/Exos) | [91] |
| Mesenchymal stem cell-derived apoptotic bodies | Endometrial regeneration and fertility restoration in rats with repair of endometrial injury | Induce macrophage immune regulation, cell proliferation and angiogenesis, effectively reduce fibrosis and promote endometrial regeneration, thereby restoring fertility | hyaluronic acid (HA) hydrogel | [95] |
| Mesenchymal stem cell-secretome (MSC-Sec) | Promotes endometrial proliferation and promotes angiogenesis | Improves the implantation environment, controls the release of various cytokines and chemokines, and promotes tissue repair and regeneration | Hyaluronic acid gel | [96,172] |
5. Outlook
Since their function was discovered, therapies for endometrial injury have involved various types of stem cells, mechanisms of action, application methods, and multifunctional platforms. This therapy can also be combined with the specific pathological characteristics of endometrial injury to individualize the treatment of other diseases (such as brain injury, liver and kidney injury, spinal cord injury, etc.).
In addition, stem cell therapy may also have a synergistic effect in combination with other treatments. Acupuncture, moxibustion, cupping, acupoint sticking, meridian acupoint massage, and other methods commonly used in traditional Chinese medicine have gradually achieved good auxiliary effects in the treatment of different diseases. In recent years, it has been reported that acupuncture can improve the receptivity of endometrium and promote the formation of pinopodes, leading to increased levels of integrin αvβ3, homeobox A10 (HOXA10), heparin-binding EGF-like growth factor (HBEGF), estrogen receptor alpha (ERα), and progesterone receptor (PR) [173]. Moreover, the effectiveness of electroacupuncture (EA) in assisted reproduction has also been confirmed [[174], [175], [176]]. For instance, It has been reported that EA can regulate uterine microcirculation [177], upregulate the expression of estrogen receptor (ER) and progesterone receptor (PR) on the surface of the endometrium, increase the levels of serum estrogen, promote endometrial regeneration [178], improve clinical pregnancy and live birth rate [179], and alleviate pain and anxiety during embryo transfer [180]. Studies have also shown that EA can stimulate the proliferation and activation of stem cells, thereby further promoting the migration of stem cells to the damaged area after tissue injury [181]. In addition, EA has been also reported to promote the migration of transplanted BMSCs and enhance their paracrine effects, and the combination of these two functions offers a promising therapeutic strategy for the treatment of endometrial injury [182,183].
So far, stem cell therapy has become a new hope for the treatment of many incurable diseases, but still faces problems such as low engraftment rate, limited efficacy, tumor formation, storage, and transportation [[184], [185], [186], [187]]. Moreover, the sources of stem cells are very limited, and the acquisition process often causes great damage or adverse effects on the psychological and physiological conditions of recipients and donors [188]. In addition, there are huge individual differences in the proliferation, survival, differentiation, and paracrine capacity of stem cells in vitro [189,190], which makes it difficult to unify and standardize the research results as a guideline for clinical treatment, thus limiting their clinical application in the treatment of IUA. Therefore, some more sources of stem cells, especially autologous MenSCs, UC-MSCs and ADSCs, have been developed [191]. According to the pathological characteristics and therapeutic requirements of IUA, functional bioscaffolds and multifunctional platforms have also begun to play an important role in the application of stem cells. In conclusion, stem cell therapy is expected to find more and more applications in the treatment of various diseases in the future.
Data availability
The data that supports this study is available from the authors upon reasonable request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The work was supported by the Key Research & Development and Promotion Project of Henan Province (212102310042, 222102310024, 222102310453), and the Basic Research Project of Key Scientific Research Projects of Colleges and Universities in Henan Province (20zx011).
Contributor Information
Shaofeng Duan, Email: sduan@henu.edu.cn.
Yanan Du, Email: duyanan@tsinghua.edu.cn.
Yuqi Guo, Email: yuqiguo@zzu.edu.cn.
References
- 1.Deans R., Abbott J. Review of intrauterine adhesions. J. Minim. Invasive Gynecol. 2010;17:555–569. doi: 10.1016/j.jmig.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 2.Xu L., Ding L., Wang L., Cao Y., Zhu H., Lu J., Li X.a., Song T., Hu Y., Dai J. Umbilical cord-derived mesenchymal stem cells on scaffolds facilitate collagen degradation via upregulation of MMP-9 in rat uterine scars. Stem Cell Res. Ther. 2017;8:84. doi: 10.1186/s13287-017-0535-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orçan S., Seven A., Isık H., Timur H., Caydere M., Ustün H., Batıoglu S. Resveratrol inhibits postoperative adhesion formation in a rat uterine horn adhesion model. Hum. Fertil. 2012;15:217–220. doi: 10.3109/14647273.2012.717337. [DOI] [PubMed] [Google Scholar]
- 4.Miwa I., Tamura H., Takasaki A., Yamagata Y., Shimamura K., Sugino N. Pathophysiologic features of "thin" endometrium. Fertil. Steril. 2009;91:998–1004. doi: 10.1016/j.fertnstert.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 5.Takasaki A., Tamura H., Miwa I., Taketani T., Shimamura K., Sugino N. Endometrial growth and uterine blood flow: a pilot study for improving endometrial thickness in the patients with a thin endometrium. Fertil. Steril. 2010;93:1851–1858. doi: 10.1016/j.fertnstert.2008.12.062. [DOI] [PubMed] [Google Scholar]
- 6.Schlaff W.D., Hurst B.S. Preoperative sonographic measurement of endometrial pattern predicts outcome of surgical repair in patients with severe Asherman's syndrome. Fertil. Steril. 1995;63:410–413. doi: 10.1016/s0015-0282(16)57379-8. [DOI] [PubMed] [Google Scholar]
- 7.Suginami K., Sato Y., Horie A., Matsumoto H., Kyo S., Araki Y., Konishi I., Fujiwara H. Platelets are a possible regulator of human endometrial re-epithelialization during menstruation. Am. J. Reprod. Immunol. 2017;77 doi: 10.1111/aji.12609. [DOI] [PubMed] [Google Scholar]
- 8.Morelli S.S., Yi P., Goldsmith L.T. Endometrial stem cells and reproduction. Obstet. Gynecol. Int. 2012;2012 doi: 10.1155/2012/851367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y., Lin X., Dai Y., Hu X., Zhu H., Jiang Y., Zhang S. Endometrial stem cells repair injured endometrium and induce angiogenesis via AKT and ERK pathways. Reproduction. 2016;152:389–402. doi: 10.1530/rep-16-0286. [DOI] [PubMed] [Google Scholar]
- 10.Goulopoulou S., Hannan J.L., Matsumoto T., Webb R.C. Pregnancy reduces RhoA/Rho kinase and protein kinase C signaling pathways downstream of thromboxane receptor activation in the rat uterine artery. Am. J. Physiol. Heart Circ. Physiol. 2012;302:H2477–H2488. doi: 10.1152/ajpheart.00900.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.March C.M. Management of Asherman's syndrome. Reprod. Biomed. Online. 2011;23:63–76. doi: 10.1016/j.rbmo.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 12.Weckstein L.N., Jacobson A., Galen D., Hampton K., Hammel J. Low-dose aspirin for oocyte donation recipients with a thin endometrium: prospective, randomized study. Fertil. Steril. 1997;68:927–930. doi: 10.1016/s0015-0282(97)00330-0. [DOI] [PubMed] [Google Scholar]
- 13.Lin X., Wei M., Li T.C., Huang Q., Huang D., Zhou F., Zhang S. A comparison of intrauterine balloon, intrauterine contraceptive device and hyaluronic acid gel in the prevention of adhesion reformation following hysteroscopic surgery for Asherman syndrome: a cohort study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013;170:512–516. doi: 10.1016/j.ejogrb.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 14.Xu B., Cao Y., Zheng Z., Galan E.A., Hu Z., Ge J., Xing X., Ma S. Injectable mesenchymal stem cell-laden matrigel microspheres for endometrium repair and regeneration. Adv. Biol. 2021;5 doi: 10.1002/adbi.202000202. [DOI] [PubMed] [Google Scholar]
- 15.Mezey E., Key S., Vogelsang G., Szalayova I., Lange G.D., Crain B. Transplanted bone marrow generates new neurons in human brains. Proc. Natl. Acad. Sci. U.S.A. 2003;100:1364–1369. doi: 10.1073/pnas.0336479100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang P., Tang X., Wang H., Dai C., Su J., Zhu H., Song M., Liu J., Nan Z., Ru T., Li Y., Wang J., Yang J., Chen B., Dai J., Hu Y. Collagen-binding basic fibroblast growth factor improves functional remodeling of scarred endometrium in uterine infertile women: a pilot study. Sci. China Life Sci. 2019;62:1617–1629. doi: 10.1007/s11427-018-9520-2. [DOI] [PubMed] [Google Scholar]
- 17.Cervelló I., Mas A., Gil-Sanchis C., Simón C. Somatic stem cells in the human endometrium. Semin. Reprod. Med. 2013;31:69–76. doi: 10.1055/s-0032-1331800. [DOI] [PubMed] [Google Scholar]
- 18.Gargett C.E., Schwab K.E., Deane J.A. Endometrial stem/progenitor cells: the first 10 years. Hum. Reprod. Update. 2016;22:137–163. doi: 10.1093/humupd/dmv051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baggish M.S., Pauerstein C.J., Woodruff J.D. Role of stroma in regeneration of endometrial epithelium. Am. J. Obstet. Gynecol. 1967;99:459–465. doi: 10.1016/0002-9378(67)90291-8. [DOI] [PubMed] [Google Scholar]
- 20.Ouyang X., You S., Zhang Y., Zhang C., Zhang G., Shao X., He F., Hu L. Transplantation of human amnion epithelial cells improves endometrial regeneration in rat model of intrauterine adhesions. Stem Cell. Dev. 2020;29:1346–1362. doi: 10.1089/scd.2019.0246. [DOI] [PubMed] [Google Scholar]
- 21.Tersoglio A.E., Tersoglio S., Salatino D.R., Castro M.a., Gonzalez A., Hinojosa M., Castellano O. Regenerative therapy by endometrial mesenchymal stem cells in thin endometrium with repeated implantation failure. Novel Strat. JBRA Assist. Reprod. 2020;24:118–127. doi: 10.5935/1518-0557.20190061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sapozhak I.M., Gubar О.S., Rodnichenko A.E., Zlatska A.V. Application of autologous endometrial mesenchymal stromal/stem cells increases thin endometrium receptivity: a case report. J. Med. Case Rep. 2020;14:190. doi: 10.1186/s13256-020-02515-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bu S., Wang Q., Zhang Q., Sun J., He B., Xiang C., Liu Z., Lai D. Human endometrial mesenchymal stem cells exhibit intrinsic anti-tumor properties on human epithelial ovarian cancer cells. Sci. Rep. 2016;6 doi: 10.1038/srep37019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan J., Li P., Wang Q., Li Y., Li X., Zhao D., Xu X., Kong L. Autologous menstrual blood-derived stromal cells transplantation for severe Asherman's syndrome. Hum. Reprod. 2016;31:2723–2729. doi: 10.1093/humrep/dew235. [DOI] [PubMed] [Google Scholar]
- 25.Ma H., Liu M., Li Y., Wang W., Yang K., Lu L., He M., Deng T., Li M., Wu D. Intrauterine transplantation of autologous menstrual blood stem cells increases endometrial thickness and pregnancy potential in patients with refractory intrauterine adhesion. J. Obstet. Gynaecol. Res. 2020;46:2347–2355. doi: 10.1111/jog.14449. [DOI] [PubMed] [Google Scholar]
- 26.Zhu H., Jiang Y., Pan Y., Shi L., Zhang S. Human menstrual blood-derived stem cells promote the repair of impaired endometrial stromal cells by activating the p38 MAPK and AKT signaling pathways. Reprod. Biol. 2018;18:274–281. doi: 10.1016/j.repbio.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 27.Zhu H., Pan Y., Jiang Y., Li J., Zhang Y., Zhang S. Activation of the Hippo/TAZ pathway is required for menstrual stem cells to suppress myofibroblast and inhibit transforming growth factor β signaling in human endometrial stromal cells. Hum. Reprod. 2019;34:635–645. doi: 10.1093/humrep/dez001. [DOI] [PubMed] [Google Scholar]
- 28.Santamaria X., Cabanillas S., Cervelló I., Arbona C., Raga F., Ferro J., Palmero J., Remohأ J., Pellicer A., Simón C. Autologous cell therapy with CD133+ bone marrow-derived stem cells for refractory Asherman's syndrome and endometrial atrophy: a pilot cohort study. Hum. Reprod. 2016;31:1087–1096. doi: 10.1093/humrep/dew042. [DOI] [PubMed] [Google Scholar]
- 29.Singh N., Shekhar B., Mohanty S., Kumar S., Seth T., Girish B. Autologous bone marrow-derived stem cell therapy for asherman's syndrome and endometrial atrophy: a 5-year follow-up study. J. Hum. Reprod. Sci. 2020;13:31–37. doi: 10.4103/jhrs.JHRS_64_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao L., Huang Z., Lin H., Tian Y., Li P., Lin S. Bone marrow mesenchymal stem cells (BMSCs) restore functional endometrium in the rat model for severe asherman syndrome. Reprod. Sci. 2019;26:436–444. doi: 10.1177/1933719118799201. [DOI] [PubMed] [Google Scholar]
- 31.Zhao G., Cao Y., Zhu X., Tang X., Ding L., Sun H., Li J., Li X., Dai C., Ru T., Zhu H., Lu J., Lin C., Wang J., Yan G., Wang H., Wang L., Dai Y., Wang B., Li R., Dai J., Zhou Y., Hu Y. Transplantation of collagen scaffold with autologous bone marrow mononuclear cells promotes functional endometrium reconstruction via downregulating ΔNp63 expression in Asherman's syndrome. Sci. China Life Sci. 2017;60:404–416. doi: 10.1007/s11427-016-0328-y. [DOI] [PubMed] [Google Scholar]
- 32.Singh N., Mohanty S., Seth T., Shankar M., Bhaskaran S., Dharmendra S. Autologous stem cell transplantation in refractory Asherman's syndrome: a novel cell based therapy. J. Hum. Reprod. Sci. 2014;7:93–98. doi: 10.4103/0974-1208.138864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cao Y., Sun H., Zhu H., Zhu X., Tang X., Yan G., Wang J., Bai D., Wang J., Wang L., Zhou Q., Wang H., Dai C., Ding L., Xu B., Zhou Y., Hao J., Dai J., Hu Y. Allogeneic cell therapy using umbilical cord MSCs on collagen scaffolds for patients with recurrent uterine adhesion: a phase I clinical trial. Stem Cell Res. Ther. 2018;9:192. doi: 10.1186/s13287-018-0904-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang J., Li Q., Yuan X., Liu Q., Zhang W., Li P. Intrauterine infusion of clinically graded human umbilical cord-derived mesenchymal stem cells for the treatment of poor healing after uterine injury: a phase I clinical trial. Stem Cell Res. Ther. 2022;13:85. doi: 10.1186/s13287-022-02756-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng J.-H., Zhang J.-K., Kong D.-S., Song Y.-B., Zhao S.-D., Qi W.-B., Li Y.-N., Zhang M.-l., Huang X.-H. Quantification of the CM-Dil-labeled human umbilical cord mesenchymal stem cells migrated to the dual injured uterus in SD rat. Stem Cell Res. Ther. 2020;11:280. doi: 10.1186/s13287-020-01806-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee S.Y., Shin J.E., Kwon H., Choi D.H., Kim J.H. Effect of autologous adipose-derived stromal vascular fraction transplantation on endometrial regeneration in patients of asherman's syndrome: a pilot study. Reprod. Sci. 2020;27:561–568. doi: 10.1007/s43032-019-00055-y. [DOI] [PubMed] [Google Scholar]
- 37.Çil N., Yaka M., Ünal M.S., Dodurga Y., Tan S., Seçme M., Karagür E.R., Mete G.A. Adipose derived mesenchymal stem cell treatment in experimental asherman syndrome induced rats. Mol. Biol. Rep. 2020;47:4541–4552. doi: 10.1007/s11033-020-05505-4. [DOI] [PubMed] [Google Scholar]
- 38.Park M., Hong S.-H., Park S.H., Kim Y.S., Yang S.C., Kim H.-R., Noh S., Na S., Lee H.K., Lim H.J., Lyu S.W., Song H. Perivascular stem cell-derived cyclophilin A improves uterine environment with asherman's syndrome via HIF1α-dependent angiogenesis. Mol. Ther. 2020;28:1818–1832. doi: 10.1016/j.ymthe.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li B., Zhang Q., Sun J., Lai D. Human amniotic epithelial cells improve fertility in an intrauterine adhesion mouse model. Stem Cell Res. Ther. 2019;10:257. doi: 10.1186/s13287-019-1368-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Azizi R., Aghebati-Maleki L., Nouri M., Marofi F., Negargar S., Yousefi M. Stem cell therapy in Asherman syndrome and thin endometrium: stem cell- based therapy. Biomed. Pharmacother. 2018;102:333–343. doi: 10.1016/j.biopha.2018.03.091. [DOI] [PubMed] [Google Scholar]
- 41.Xu S., Chan R.W.S., Ng E.H.Y., Yeung W.S.B. Spatial and temporal characterization of endometrial mesenchymal stem-like cells activity during the menstrual cycle. Exp. Cell Res. 2017;350:184–189. doi: 10.1016/j.yexcr.2016.11.020. [DOI] [PubMed] [Google Scholar]
- 42.Ai J., Shahverdi A.R., Barough S.E., Kouchesfehani H.M., Heidari S., Roozafzoon R., Verdi J., Khoshzaban A. Derivation of adipocytes from human endometrial stem cells (EnSCs) J. Reorod. Infertil. 2012;13:151–157. [PMC free article] [PubMed] [Google Scholar]
- 43.Wolff E.F., Wolff A.B., Du H., Taylor H.S. Demonstration of multipotent stem cells in the adult human endometrium by in vitro chondrogenesis. Reprod. Sci. 2007;14:524–533. doi: 10.1177/1933719107306896. [DOI] [PubMed] [Google Scholar]
- 44.Su K., Edwards S.L., Tan K.S., White J.F., Kandel S., Ramshaw J.A.M., Gargett C.E., Werkmeister J.A. Induction of endometrial mesenchymal stem cells into tissue-forming cells suitable for fascial repair. Acta Biomater. 2014;10:5012–5020. doi: 10.1016/j.actbio.2014.08.031. [DOI] [PubMed] [Google Scholar]
- 45.Ikegami Y., Miyoshi S., Nishiyama N., Hida N., Okamoto K., Miyado K., Segawa K., Ogawa S., Umezawa A. Serum-independent cardiomyogenic transdifferentiation in human endometrium-derived mesenchymal cells. Artif. Organs. 2010;34:280–288. doi: 10.1111/j.1525-1594.2009.00859.x. [DOI] [PubMed] [Google Scholar]
- 46.Mobarakeh Z.T., Ai J., Yazdani F., Sorkhabadi S.M.R., Ghanbari Z., Javidan A.N., Mortazavi-Tabatabaei S.A., Massumi M., Barough S.E. Human endometrial stem cells as a new source for programming to neural cells. Cell Biol. Int. Rep. 2012;19 doi: 10.1042/cbr20110009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mutlu L., Hufnagel D., Taylor H.S. The endometrium as a source of mesenchymal stem cells for regenerative medicine. Biol. Reprod. 2015;92:138. doi: 10.1095/biolreprod.114.126771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Phermthai T., Tungprasertpol K., Julavijitphong S., Pokathikorn P., Thongbopit S., Wichitwiengrat S. Successful derivation of xeno-free mesenchymal stem cell lines from endometrium of infertile women. Reprod. Biol. 2016;16:261–268. doi: 10.1016/j.repbio.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 49.Musina R.A., Belyavski A.V., Tarusova O.V., Solovyova E.V., Sukhikh G.T. Endometrial mesenchymal stem cells isolated from the menstrual blood. Bull. Exp. Biol. Med. 2008;145:539–543. doi: 10.1007/s10517-008-0136-0. [DOI] [PubMed] [Google Scholar]
- 50.Rodrigues M.C.O., Lippert T., Nguyen H., Kaelber S., Sanberg P.R., Borlongan C.V. Menstrual blood-derived stem cells: in vitro and in vivo characterization of functional effects. Adv. Exp. Med. Biol. 2016;951:111–121. doi: 10.1007/978-3-319-45457-3_9. [DOI] [PubMed] [Google Scholar]
- 51.Hu J., Song K., Zhang J., Zhang Y., Tan B.-Z. Effects of menstrual blood-derived stem cells on endometrial injury repair. Mol. Med. Rep. 2019;19:813–820. doi: 10.3892/mmr.2018.9744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D., Moorman M.A., Simonetti D.W., Craig S., Marshak D.R. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 53.Orlic D., Kajstura J., Chimenti S., Jakoniuk I., Anderson S.M., Li B., Pickel J., McKay R., Nadal-Ginard B., Bodine D.M., Leri A., Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 54.Sasaki M., Abe R., Fujita Y., Ando S., Inokuma D., Shimizu H. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J. Immunol. 2008;180:2581–2587. doi: 10.4049/jimmunol.180.4.2581. [DOI] [PubMed] [Google Scholar]
- 55.Cervelló I., Gil-Sanchis C., Mas A., Faus A., Sanz J., Moscardó F., Higueras G., Sanz M.A., Pellicer A., Simón C. Bone marrow-derived cells from male donors do not contribute to the endometrial side population of the recipient. PLoS One. 2012;7 doi: 10.1371/journal.pone.0030260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ikoma T., Kyo S., Maida Y., Ozaki S., Takakura M., Nakao S., Inoue M. Bone marrow-derived cells from male donors can compose endometrial glands in female transplant recipients. Am. J. Obstet. Gynecol. 2009;201 doi: 10.1016/j.ajog.2009.07.026. 608.e601-608. [DOI] [PubMed] [Google Scholar]
- 57.Mints M., Jansson M., Sadeghi B., Westgren M., Uzunel M., Hassan M., Palmblad J. Endometrial endothelial cells are derived from donor stem cells in a bone marrow transplant recipient. Hum. Reprod. 2008;23:139–143. doi: 10.1093/humrep/dem342. [DOI] [PubMed] [Google Scholar]
- 58.Wang X., Mamillapalli R., Mutlu L., Du H., Taylor H.S. Chemoattraction of bone marrow-derived stem cells towards human endometrial stromal cells is mediated by estradiol regulated CXCL12 and CXCR4 expression. Stem Cell Res. 2015;15:14–22. doi: 10.1016/j.scr.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sakr S., Naqvi H., Komm B., Taylor H.S. Endometriosis impairs bone marrow-derived stem cell recruitment to the uterus whereas bazedoxifene treatment leads to endometriosis regression and improved uterine stem cell engraftment. Endocrinology. 2014;155:1489–1497. doi: 10.1210/en.2013-1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Theise N.D., Nimmakayalu M., Gardner R., Illei P.B., Morgan G., Teperman L., Henegariu O., Krause D.S. Liver from bone marrow in humans. Hepatology. 2000;32:11–16. doi: 10.1053/jhep.2000.9124. [DOI] [PubMed] [Google Scholar]
- 61.Quaini F., Urbanek K., Beltrami A.P., Finato N., Beltrami C.A., Nadal-Ginard B., Kajstura J., Leri A., Anversa P. Chimerism of the transplanted heart. N. Engl. J. Med. 2002;346:5–15. doi: 10.1056/NEJMoa012081. [DOI] [PubMed] [Google Scholar]
- 62.Körbling M., Katz R.L., Khanna A., Ruifrok A.C., Rondon G., Albitar M., Champlin R.E., Estrov Z. Hepatocytes and epithelial cells of donor origin in recipients of peripheral-blood stem cells. N. Engl. J. Med. 2002;346:738–746. doi: 10.1056/NEJMoa3461002. [DOI] [PubMed] [Google Scholar]
- 63.Nemeth K., Key S., Bottlik G., Masszi T., Mezey E., Karpati S. Analyses of donor-derived keratinocytes in hairy and nonhairy skin biopsies of female patients following allogeneic male bone marrow transplantation. Stem Cell. Dev. 2012;21:152–157. doi: 10.1089/scd.2010.0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Taylor H.S. Endometrial cells derived from donor stem cells in bone marrow transplant recipients. JAMA. 2004;292:81–85. doi: 10.1001/jama.292.1.81. [DOI] [PubMed] [Google Scholar]
- 65.Du H., Naqvi H., Taylor H.S. Ischemia/reperfusion injury promotes and granulocyte-colony stimulating factor inhibits migration of bone marrow-derived stem cells to endometrium. Stem Cell. Dev. 2012;21:3324–3331. doi: 10.1089/scd.2011.0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang W.-J. a. r., Cheng M.-J., Huang Y.-T., Jiang W., Cong Q., Zheng Y.-F., Xu C.-J. A study in vitro on differentiation of bone marrow mesenchymal stem cells into endometrial epithelial cells in mice. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012;160:185–190. doi: 10.1016/j.ejogrb.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 67.Cervelló I., Gil-Sanchis C., Mas A., Delgado-Rosas F., Martínez-Conejero J.A., Galán A., Martínez-Romero A., Martínez S., Navarro I., Ferro J., Horcajadas J.A., Esteban F.J., O'Connor J.E., Pellicer A., Simón C. Human endometrial side population cells exhibit genotypic, phenotypic and functional features of somatic stem cells. PLoS One. 2010;5 doi: 10.1371/journal.pone.0010964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou L.N., Wang J.C., Zilundu P.L.M., Wang Y.Q., Guo W.P., Zhang S.X., Luo H., Zhou J.H., Deng R.D., Chen D.F. A comparison of the use of adipose-derived and bone marrow-derived stem cells for peripheral nerve regeneration in vitro and in vivo. Stem Cell Res. Ther. 2020;11:153. doi: 10.1186/s13287-020-01661-3. https://doi: 10.1186/s13287-020-01661-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Du H., Taylor H.S. Contribution of bone marrow-derived stem cells to endometrium and endometriosis. Stem Cell. 2007;25:2082–2086. doi: 10.1634/stemcells.2006-0828. [DOI] [PubMed] [Google Scholar]
- 70.Jing Z., Qiong Z., Yonggang W., Yanping L. Rat bone marrow mesenchymal stem cells improve regeneration of thin endometrium in rat. Fertil. Steril. 2014;101:587–594. doi: 10.1016/j.fertnstert.2013.10.053. [DOI] [PubMed] [Google Scholar]
- 71.Zhao J., Zhang Q., Wang Y., Li Y. Uterine infusion with bone marrow mesenchymal stem cells improves endometrium thickness in a rat model of thin endometrium. Reprod. Sci. 2015;22:181–188. doi: 10.1177/1933719114537715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Caras I., Shapiro B. Partial purification and properties of microsomal phosphatidate phosphohydrolase from rat liver. Biochim. Biophys. Acta. 1975;409:201–211. doi: 10.1016/0005-2760(75)90154-x. [DOI] [PubMed] [Google Scholar]
- 73.Zhu H., Hou C.-C., Luo L.-F., Hu Y.-J., Yang W.-X. Endometrial stromal cells and decidualized stromal cells: origins, transformation and functions. Gene. 2014;551:1–14. doi: 10.1016/j.gene.2014.08.047. [DOI] [PubMed] [Google Scholar]
- 74.Wu H.-Y., Zhang X.-C., Jia B.-B., Cao Y., Yan K., Li J.-Y., Tao L., Jie Z.-G., Liu Q.-W. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate acetaminophen-induced acute liver failure through activating ERK and IGF-1R/PI3K/AKT signaling pathway. J. Pharmacol. Sci. 2021;147:143–155. doi: 10.1016/j.jphs.2021.06.008. [DOI] [PubMed] [Google Scholar]
- 75.Tobita M., Orbay H., Mizuno H. Adipose-derived stem cells: current findings and future perspectives. Discov. Med. 2011;11:160–170. [PubMed] [Google Scholar]
- 76.Mizuno H. Adipose-derived stem cells for regenerative medicine in the field of plastic and reconstructive surgery. J. Oral Biosci. 2013;55:132–136. doi: 10.1016/j.job.2013.04.005. [DOI] [Google Scholar]
- 77.Zhang Y., Khan D., Delling J., Tobiasch E. Mechanisms underlying the osteo- and adipo-differentiation of human mesenchymal stem cells. Sci. World J. 2012;2012 doi: 10.1100/2012/793823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zuk P.A., Zhu M., Mizuno H., Huang J., Futrell J.W., Katz A.J., Benhaim P., Lorenz H.P., Hedrick M.H. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 79.Gomillion C.T., Burg K.J.L. Stem cells and adipose tissue engineering. Biomaterials. 2006;27:6052–6063. doi: 10.1016/j.biomaterials.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 80.Cheung H.K., Han T.T.Y., Marecak D.M., Watkins J.F., Amsden B.G., Flynn L.E. Composite hydrogel scaffolds incorporating decellularized adipose tissue for soft tissue engineering with adipose-derived stem cells. Biomaterials. 2014;35:1914–1923. doi: 10.1016/j.biomaterials.2013.11.067. [DOI] [PubMed] [Google Scholar]
- 81.Shekkeris A.S., Jaiswal P.K., Khan W.S. Clinical applications of mesenchymal stem cells in the treatment of fracture non-union and bone defects. Curr. Stem Cell Res. Ther. 2012;7:127–133. doi: 10.2174/157488812799218956. [DOI] [PubMed] [Google Scholar]
- 82.Melief S.M., Zwaginga J.J., Fibbe W.E., Roelofs H. Adipose tissue-derived multipotent stromal cells have a higher immunomodulatory capacity than their bone marrow-derived counterparts. Stem Cells Transl. Med. 2013;2:455–463. doi: 10.5966/sctm.2012-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gargett C.E., Ye L. Endometrial reconstruction from stem cells. Fertil. Steril. 2012;98:11–20. doi: 10.1016/j.fertnstert.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 84.Sanchez-Mata A., Gonzalez-Muñoz E. Understanding menstrual blood-derived stromal/stem cells: definition and properties. Are we rushing into their therapeutic applications? iScience. 2021;24 doi: 10.1016/j.isci.2021.103501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu Y., Niu R., Li W., Lin J., Stamm C., Steinhoff G., Ma N. Therapeutic potential of menstrual blood-derived endometrial stem cells in cardiac diseases. Cell. Mol. Life Sci. 2019;76:1681–1695. doi: 10.1007/s00018-019-03019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bozorgmehr M., Gurung S., Darzi S., Nikoo S., Kazemnejad S., Zarnani A.-H., Gargett C.E. Endometrial and menstrual blood mesenchymal stem/stromal cells: biological properties and clinical application. Front. Cell Dev. Biol. 2020;8:497. doi: 10.3389/fcell.2020.00497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gurtner G.C., Werner S., Barrandon Y., Longaker M.T. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 88.Tan Q., Xia D., Ying X. miR-29a in exosomes from bone marrow mesenchymal stem cells inhibit fibrosis during endometrial repair of intrauterine adhesion. Int. J. Stem Cells. 2020;13:414–423. doi: 10.15283/ijsc20049. https://doi: 10.15283/ijsc20049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shi Q., Wang D., Ding X., Yang X., Zhang Y. Exosome-shuttled miR-7162-3p from human umbilical cord derived mesenchymal stem cells repair endometrial stromal cell injury by restricting APOL6. Arch. Biochem. Biophys. 2021;707 doi: 10.1016/j.abb.2021.108887. https://doi:10.1016/j.abb.2021.108887 [DOI] [PubMed] [Google Scholar]
- 90.Lin J., Wang Z., Huang J., Tang S., Saiding Q., Zhu Q., Cui W. Microenvironment-protected exosome-hydrogel for facilitating endometrial regeneration, fertility restoration, and live birth of offspring. Small. 2021;17 doi: 10.1002/smll.202007235. https://doi: 10.1002/smll.202007235 [DOI] [PubMed] [Google Scholar]
- 91.Xin L., Lin X., Zhou F., Li C., Wang X., Yu H., Pan Y., Fei H., Ma L., Zhang S. A scaffold laden with mesenchymal stem cell-derived exosomes for promoting endometrium regeneration and fertility restoration through macrophage immunomodulation. Acta Biomater. 2020;113:252–266. doi: 10.1016/j.actbio.2020.06.029. [DOI] [PubMed] [Google Scholar]
- 92.Zhang L., Li Y., Guan C.-Y., Tian S., Lv X.-D., Li J.-H., Ma X., Xia H.-F. Therapeutic effect of human umbilical cord-derived mesenchymal stem cells on injured rat endometrium during its chronic phase. Stem Cell Res. Ther. 2018;9:36. doi: 10.1186/s13287-018-0777-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Panagiotopoulou N., Karavolos S., Choudhary M. Endometrial injury prior to assisted reproductive techniques for recurrent implantation failure: a systematic literature review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015;193:27–33. doi: 10.1016/j.ejogrb.2015.06.026. [DOI] [PubMed] [Google Scholar]
- 94.Aflatoonian A., Bagheri R.B., Hosseinisadat R. The effect of endometrial injury on pregnancy rate in frozen-thawed embryo transfer: a randomized control trial. Int. J. Reprod. Biomed. (Yazd) 2016;14:453–458. doi: 10.18502/ijrm.v20i3.10715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xin L., Wei C., Tong X., Dai Y., Huang D., Chen J., Ma L., Zhang S. In situ delivery of apoptotic bodies derived from mesenchymal stem cells via a hyaluronic acid hydrogel: a therapy for intrauterine adhesions. Bioact. Mater. 2022;12:107–119. doi: 10.1016/j.bioactmat.2021.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu F., Hu S., Yang H., Li Z., Huang K., Su T., Wang S., Cheng K. Hyaluronic acid hydrogel integrated with mesenchymal stem cell-secretome to treat endometrial injury in a rat model of asherman's syndrome. Adv. Healthcare Mater. 2019;8 doi: 10.1002/adhm.201900411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang C., Shang Y., Chen X., Midgley A.C., Wang Z., Zhu D., Wu J., Chen P., Wu L., Wang X., Zhang K., Wang H., Kong D., Yang Z., Li Z., Chen X. Supramolecular nanofibers containing arginine-glycine-aspartate (RGD) peptides boost therapeutic efficacy of extracellular vesicles in kidney repair. ACS Nano. 2020;14:12133–12147. doi: 10.1021/acsnano.0c05681. [DOI] [PubMed] [Google Scholar]
- 98.Rao D., Huang D., Sang C., Zhong T., Zhang Z., Tang Z. Advances in mesenchymal stem cell-derived exosomes as drug delivery vehicles. Front. Bioeng. Biotechnol. 2022;4 doi: 10.3389/fbioe.2021.797359. https://doi: 10.3389/fbioe.2021.797359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kollet O., Shivtiel S., Chen Y.-Q., Suriawinata J., Thung S.N., Dabeva M.D., Kahn J., Spiegel A., Dar A., Samira S., Goichberg P., Kalinkovich A., Arenzana-Seisdedos F., Nagler A., Hardan I., Revel M., Shafritz D.A., Lapidot T. HGF, SDF-1, and MMP-9 are involved in stress-induced human CD34+ stem cell recruitment to the liver. J. Clin. Invest. 2003;112:160–169. doi: 10.1172/jci17902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wright D.E., Bowman E.P., Wagers A.J., Butcher E.C., Weissman I.L. Hematopoietic stem cells are uniquely selective in their migratory response to chemokines. J. Exp. Med. 2002;195:1145–1154. doi: 10.1084/jem.20011284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sisto M., Ribatti D., Lisi S. SMADS-mediate molecular mechanisms in sjögren's syndrome. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22063203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu R.M., Pravia K. Oxidative stress and glutathione in TGF-beta-mediated fibrogenesis. Free Radic. Biol. Med. 2010;48:1–15. doi: 10.1016/j.freeradbiomed.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Galleu A., Riffo-Vasquez Y., Trento C., Lomas C., Dolcetti L., Cheung T.S., Bonin M.v., Barbieri L., Halai K., Ward S., Weng L., Chakraverty R., Lombardi G., Watt F.M., Orchard K., Marks D.I., Apperley J., Bornhauser M., Walczak H., Bennett C., Dazzi F. Apoptosis in mesenchymal stromal cells induces in vivo recipient-mediated immunomodulation. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aam7828. [DOI] [PubMed] [Google Scholar]
- 104.Wang J., Ju B., Pan C., Gu Y., Zhang Y., Sun L., Zhang B., Zhang Y. Application of bone marrow-derived mesenchymal stem cells in the treatment of intrauterine adhesions in rats. Cell. Physiol. Biochem. 2016;39:1553–1560. doi: 10.1159/000447857. [DOI] [PubMed] [Google Scholar]
- 105.Gao L., Huang Z., Lin H., Tian Y., Li P., Lin S. Bone marrow mesenchymal stem cells (BMSCs) restore functional endometrium in the rat model for severe asherman syndrome. Reprod. Sci. 2019;26:436–444. doi: 10.1177/1933719118799201. [DOI] [PubMed] [Google Scholar]
- 106.Nagori C.B., Panchal S.Y., Patel H. Endometrial regeneration using autologous adult stem cells followed by conception by in vitro fertilization in a patient of severe Asherman's syndrome. J. Hum. Reprod. Sci. 2011;4:43–48. doi: 10.4103/0974-1208.82360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen L., Guo L., Chen F., Xie Y., Zhang H., Quan P., Sui L. Transplantation of menstrual blood-derived mesenchymal stem cells (MbMSCs) promotes the regeneration of mechanical injuried endometrium. Am. J. Transl. Res. 2020;12:4941–4954. [PMC free article] [PubMed] [Google Scholar]
- 108.Kou L., Jiang X., Xiao S., Zhao Y.Z., Yao Q., Chen R. Therapeutic options and drug delivery strategies for the prevention of intrauterine adhesions. J. Contr. Release. 2020;318:25–37. doi: 10.1016/j.jconrel.2019.12.007. https://doi: 10.1016/j.jconrel.2019.12.007 [DOI] [PubMed] [Google Scholar]
- 109.Martino S., D'Angelo F., Armentano I., Kenny J.M., Orlacchio A. Stem cell-biomaterial interactions for regenerative medicine. Biotechnol. Adv. 2012;30:338–351. doi: 10.1016/j.biotechadv.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 110.Bergman R.A. Uterine smooth muscle fibers in castrate and estrogen-treated rats. J. Cell Biol. 1968;36:639–648. doi: 10.1083/jcb.36.3.639. https://doi: 10.1083/jcb.36.3.639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang J., Yang C., Xie Y., Chen X., Jiang T., Tian J., Hu S., Lu Y. Application of bioactive hydrogels for functional treatment of intrauterine adhesion. Front. Bioeng. Biotechnol. 2021;9 doi: 10.3389/fbioe.2021.760943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Stratton S., Shelke N.B., Hoshino K., Rudraiah S., Kumbar S.G. Bioactive polymeric scaffolds for tissue engineering. Bioact. Mater. 2016;1:93–108. doi: 10.1016/j.bioactmat.2016.11.001. https://doi: 10.1016/j.bioactmat.2016.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jung Y.S., Park W., Park H., Lee D.K., Na K. Thermo-sensitive injectable hydrogel based on the physical mixing of hyaluronic acid and Pluronic F-127 for sustained NSAID delivery. Carbohydr. Polym. 2017;156:403–408. doi: 10.1016/j.carbpol.2016.08.068. https://doi: 10.1016/j.carbpol.2016.08.068 [DOI] [PubMed] [Google Scholar]
- 114.Yang H., Wu S., Feng R., Huang J., Liu L., Liu F., Chen Y. Vitamin C plus hydrogel facilitates bone marrow stromal cell-mediated endometrium regeneration in rats. Stem Cell Res. Ther. 2017;8:267. doi: 10.1186/s13287-017-0718-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yao Q., Zheng Y.-W., Lan Q.-H., Wang L.-F., Huang Z.-W., Chen R., Yang Y., Xu H.-L., Kou L., Zhao Y.-Z. Aloe/poloxamer hydrogel as an injectable β-estradiol delivery scaffold with multi-therapeutic effects to promote endometrial regeneration for intrauterine adhesion treatment. Eur. J. Pharmaceut. Sci. 2020;148 doi: 10.1016/j.ejps.2020.105316. [DOI] [PubMed] [Google Scholar]
- 116.Xu H.-L., Xu J., Zhang S.-S., Zhu Q.-Y., Jin B.-H., ZhuGe D.-L., Shen B.-X., Wu X.-Q., Xiao J., Zhao Y.-Z. Temperature-sensitive heparin-modified poloxamer hydrogel with affinity to KGF facilitate the morphologic and functional recovery of the injured rat uterus. Drug Deliv. 2017;24:867–881. doi: 10.1080/10717544.2017.1333173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Xiao B., Yang W., Lei D., Huang J., Yin Y., Zhu Y., You Z., Wang F., Sun S. PGS scaffolds promote the in vivo survival and directional differentiation of bone marrow mesenchymal stem cells restoring the morphology and function of wounded rat uterus. Adv. Healthcare Mater. 2019;8 doi: 10.1002/adhm.201801455. [DOI] [PubMed] [Google Scholar]
- 118.Wang B., Feng C., Dang J., Zhu Y., Yang X., Zhang T., Zhang R., Li J., Tang J., Shen C., Shen L., Dong J., Zhang X. Preparation of fibroblast suppressive poly(ethylene glycol)-b-poly(l-phenylalanine)/Poly(ethylene glycol) hydrogel and its application in intrauterine fibrosis prevention. ACS Biomater. Sci. Eng. 2021;7:311–321. doi: 10.1021/acsbiomaterials.0c01390. [DOI] [PubMed] [Google Scholar]
- 119.Han Y., Liu S., Mao H., Tian L., Ning W. Synthesis of novel temperature- and pH-sensitive ABA triblock copolymers P(DEAEMA-co-MEO₂MA-co-OEGMA) -b-PEG-b-P (DEAEMA-co- MEO₂MA-co-OEGMA):Micellization, Sol⁻Gel transitions, and sustained BSA release. Polymers. 2016;8 doi: 10.3390/polym8110367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wu Y., Xiang Y., Fang J., Li X., Lin Z., Dai G., Yin J., Wei P., Zhang D. The influence of the stiffness of GelMA substrate on the outgrowth of PC12 cells. Biosci. Rep. 2019;39 doi: 10.1042/bsr20181748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Feng M., Hu S., Qin W., Tang Y., Guo R., Han L. Bioprinting of a blue light-cross-linked biodegradable hydrogel encapsulating amniotic mesenchymal stem cells for intrauterine adhesion prevention. ACS Omega. 2021;6:23067–23075. doi: 10.1021/acsomega.1c02117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Surgery A.E.G. AAGL practice report: practice guidelines on intrauterine adhesions developed in collaboration with the European Society of Gynaecological Endoscopy (ESGE) Gynecol. Surg. 2017;14:6. doi: 10.1186/s10397-017-1007-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang L., Yu C., Chang T., Zhang M., Song S., Xiong C., Su P., Xiang W. In situ repair abilities of human umbilical cord-derived mesenchymal stem cells and autocrosslinked hyaluronic acid gel complex in rhesus monkeys with intrauterine adhesion. Sci. Adv. 2020;6 doi: 10.1126/sciadv.aba6357. eaba6357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kim Y.Y., Park K.-H., Kim Y.J., Kim M.S., Liu H.C., Rosenwaks Z., Ku S.-Y. Synergistic regenerative effects of functionalized endometrial stromal cells with hyaluronic acid hydrogel in a murine model of uterine damage. Acta Biomater. 2019;89:139–151. doi: 10.1016/j.actbio.2019.03.032. [DOI] [PubMed] [Google Scholar]
- 125.Movahedi M., Asefnejad A., Rafienia M., Khorasani M.T. Potential of novel electrospun core-shell structured polyurethane/starch (hyaluronic acid) nanofibers for skin tissue engineering: in vitro and in vivo evaluation. Int. J. Biol. Macromol. 2020;146:627–637. doi: 10.1016/j.ijbiomac.2019.11.233. [DOI] [PubMed] [Google Scholar]
- 126.Chen Z.G., Wang P.W., Wei B., Mo X.M., Cui F.Z. Electrospun collagen-chitosan nanofiber: a biomimetic extracellular matrix for endothelial cell and smooth muscle cell. Acta Biomater. 2010;6:372–382. doi: 10.1016/j.actbio.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 127.Kong M., Chen X.G., Xing K., Park H.J. Antimicrobial properties of chitosan and mode of action: a state of the art review. Int. J. Food Microbiol. 2010;144:51–63. doi: 10.1016/j.ijfoodmicro.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 128.Shamosi A., Mehrabani D., Azami M., Ebrahimi-Barough S., Siavashi V., Ghanbari H., Sharifi E., Roozafzoon R., Ai J. Differentiation of human endometrial stem cells into endothelial-like cells on gelatin/chitosan/bioglass nanofibrous scaffolds. Artif. Cell Nanomed. Biotechnol. 2017;45:163–173. doi: 10.3109/21691401.2016.1138493. [DOI] [PubMed] [Google Scholar]
- 129.Zhang E., Guo Q., Ji F., Tian X., Cui J., Song Y., Sun H., Li J., Yao F. Thermoresponsive polysaccharide-based composite hydrogel with antibacterial and healing-promoting activities for preventing recurrent adhesion after adhesiolysis. Acta Biomater. 2018;74:439–453. doi: 10.1016/j.actbio.2018.05.037. [DOI] [PubMed] [Google Scholar]
- 130.Yan X., Chen B., Lin Y., Li Y., Xiao Z., Hou X., Tan Q., Dai J. Acceleration of diabetic wound healing by collagen-binding vascular endothelial growth factor in diabetic rat model. Diabetes Res. Clin. Pract. 2010;90:66–72. doi: 10.1016/j.diabres.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 131.Zhao Y., Zhang J., Wang X., Chen B., Xiao Z., Shi C., Wei Z., Hou X., Wang Q., Dai J. The osteogenic effect of bone morphogenetic protein-2 on the collagen scaffold conjugated with antibodies. J. Contr. Release. 2010;141:30–37. doi: 10.1016/j.jconrel.2009.06.032. [DOI] [PubMed] [Google Scholar]
- 132.Boccafoschi F., Habermehl J., Vesentini S., Mantovani D. Biological performances of collagen-based scaffolds for vascular tissue engineering. Biomaterials. 2005;26:7410–7417. doi: 10.1016/j.biomaterials.2005.05.052. [DOI] [PubMed] [Google Scholar]
- 133.Ahmed M.R., Vairamuthu S., Shafiuzama M., Basha S.H., Jayakumar R. Microwave irradiated collagen tubes as a better matrix for peripheral nerve regeneration. Brain Res. 2005;1046:55–67. doi: 10.1016/j.brainres.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 134.Ding L., Li X.a., Sun H., Su J., Lin N., Péault B., Song T., Yang J., Dai J., Hu Y. Transplantation of bone marrow mesenchymal stem cells on collagen scaffolds for the functional regeneration of injured rat uterus. Biomaterials. 2014;35:4888–4900. doi: 10.1016/j.biomaterials.2014.02.046. [DOI] [PubMed] [Google Scholar]
- 135.Kathuria N., Tripathi A., Kar K.K., Kumar A. Synthesis and characterization of elastic and macroporous chitosan-gelatin cryogels for tissue engineering. Acta Biomater. 2009;5:406–418. doi: 10.1016/j.actbio.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 136.Ji W., Hou B., Lin W., Wang L., Zheng W., Li W., Zheng J., Wen X., He P. 3D Bioprinting a human iPSC-derived MSC-loaded scaffold for repair of the uterine endometrium. Acta Biomater. 2020;116:268–284. doi: 10.1016/j.actbio.2020.09.012. [DOI] [PubMed] [Google Scholar]
- 137.Asai D., Xu D., Liu W., Quiroz F.G., Callahan D.J., Zalutsky M.R., Craig S.L., Chilkoti A. Protein polymer hydrogels by in situ, rapid and reversible self-gelation. Biomaterials. 2012;33:5451–5458. doi: 10.1016/j.biomaterials.2012.03.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Geckil H., Xu F., Zhang X., Moon S., Demirci U. Engineering hydrogels as extracellular matrix mimics. Nanomedicine. 2010;5:469–484. doi: 10.2217/nnm.10.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Willerth S.M., Sakiyama-Elbert S.E. StemBook; 2008. Combining Stem Cells and Biomaterial Scaffolds for Constructing Tissues and Cell Delivery. [PubMed] [Google Scholar]
- 140.Shang X.-L., Tao H.-Y., Chen S.-Y., Li Y.-X., Hua Y.-H. Clinical and MRI outcomes of HA injection following arthroscopic microfracture for osteochondral lesions of the talus. Knee Surg. Sports Traumatol. Arthrosc. 2016;24:1243–1249. doi: 10.1007/s00167-015-3575-y. [DOI] [PubMed] [Google Scholar]
- 141.Park S.-R., Kim S.-R., Im J.B., Park C.H., Lee H.-Y., Hong I.-S. 3D stem cell-laden artificial endometrium: successful endometrial regeneration and pregnancy. Biofabrication. 2021;13 doi: 10.1088/1758-5090/ac165a. [DOI] [PubMed] [Google Scholar]
- 142.Cai G., Hou Z., Sun W., Li P., Zhang J., Yang L., Chen J. Recent developments in biomaterial-based hydrogel as the delivery system for repairing endometrial injury. Front. Bioeng. Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.894252. https://doi: 10.3389/fbioe.2022.894252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Park S.-R., Kim S.-R., Im J.B., Park C.H., Lee H.-Y., Hong I.-S. 3D stem cell-laden artificial endometrium: successful endometrial regeneration and pregnancy. Biofabrication. 2021;13 doi: 10.1088/1758-5090/ac165a. [DOI] [PubMed] [Google Scholar]
- 144.Damodarasamy M., Johnson R.S., Bentov I., Maccoss M.J., Vernon R.B., Reed M.J. Hyaluronan enhances wound repair and increases collagen III in aged dermal wounds. Wound Repair Regen. 2014;22:521–526. doi: 10.1111/wrr.12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mencner P. Olczyk, Komosinska-Vassev K. The role of the extracellular matrix components in cutaneous wound healing. BioMed Res. Int. 2014;2014 doi: 10.1155/2014/747584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Corrêa M.E.A.B., Mendes C., Bittencourt J.V.S., Takejima A., de Souza I.C., de Carvalho S.C.D., Orlandini I.G., de Andrade T.A.M., Guarita-Souza L.C., Silveira P.C.L. Effects of the application of decellularized amniotic membrane solubilized with hyaluronic acid on wound healing. Ann. Biomed. Eng. 2022 doi: 10.1007/s10439-022-03008-w. [DOI] [PubMed] [Google Scholar]
- 147.Chen P., Lu M., Wang T., Dian D., Zhong Y., Aleahmad M. Human amniotic membrane as a delivery vehicle for stem cell-based therapies. Life Sci. 2021;272 doi: 10.1016/j.lfs.2021.119157. [DOI] [PubMed] [Google Scholar]
- 148.Chen Y., Fei W., Zhao Y., Wang F., Zheng X., Luan X., Zheng C. Sustained delivery of 17β-estradiol by human amniotic extracellular matrix (HAECM) scaffold integrated with PLGA microspheres for endometrium regeneration. Drug Deliv. 2020;27:1165–1175. doi: 10.1080/10717544.2020.1801891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fortunati E., D'Angelo F., Martino S., Orlacchio A., Kenny J.M., Armentano I. Carbon nanotubes and silver nanoparticles for multifunctional conductive biopolymer composites. Carbon. 2011;49:2370–2379. doi: 10.1016/j.carbon.2011.02.004. [DOI] [Google Scholar]
- 150.Gupta A., Singh S. Potential role of growth factors controlled release in achieving enhanced neuronal trans-differentiation from mesenchymal stem cells for neural tissue repair and regeneration. Mol. Neurobiol. 2022;59:983–1001. doi: 10.1007/s12035-021-02646-w. [DOI] [PubMed] [Google Scholar]
- 151.Lee A.S., Inayathullah M., Lijkwan M.A., Zhao X., Sun W., Park S., Hong W.X., Parekh M.B., Malkovskiy A.V., Lau E., Qin X., Pothineni V.R., Sanchez-Freire V.n., Zhang W.Y., Kooreman N.G., Ebert A.D., Chan C.K.F., Nguyen P.K., Rajadas J., Wu J.C. Prolonged survival of transplanted stem cells after ischaemic injury via the slow release of pro-survival peptides from a collagen matrix. Nat. Biomed. Eng. 2018;2:104–113. doi: 10.1038/s41551-018-0191-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sato-Nishiuchi R., Li S., Ebisu F., Sekiguchi K. Recombinant laminin fragments endowed with collagen-binding activity: a tool for conferring laminin-like cell-adhesive activity to collagen matrices. Matrix Biol. 2018;65:75–90. doi: 10.1016/j.matbio.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 153.Feng G., Zhang J., Li Y., Nie Y., Zhu D., Wang R., Liu J., Gao J., Liu N., He N., Du W., Tao H., Che Y., Xu Y., Kong D., Zhao Q., Li Z. IGF-1 C domain-modified hydrogel enhances cell therapy for AKI. J. Am. Soc. Nephrol. 2016;28:2357–2369. doi: 10.1681/ASN.2015050578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Meng Q., Man Z., Dai L., Huang H., Zhang X., Hu X., Shao Z., Zhu J., Zhang J., Fu X., Duan X., Ao Y. A composite scaffold of MSC affinity peptide-modified demineralized bone matrix particles and chitosan hydrogel for cartilage regeneration. Sci. Rep. 2015;5 doi: 10.1038/srep17802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Liu X., Wang X., Wang X., Ren H., He J., Qiao L., Cui F.-Z. Functionalized self-assembling peptide nanofiber hydrogels mimic stem cell niche to control human adipose stem cell behavior in vitro. Acta Biomater. 2013;9:6798–6805. doi: 10.1016/j.actbio.2013.01.027. [DOI] [PubMed] [Google Scholar]
- 156.Ponomaryov T., Peled A., Petit I., Taichman R.S., Habler L., Sandbank J., Arenzana-Seisdedos F., Magerus A., Caruz A., Fujii N., Nagler A., Lahav M., Szyper-Kravitz M., Zipori D., Lapidot T. Induction of the chemokine stromal-derived factor-1 following DNA damage improves human stem cell function. J. Clin. Invest. 2000;106:1331–1339. doi: 10.1172/jci10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wenbo Q., Lijian X., Shuangdan Z., Jiahua Z., Yanpeng T., Xuejun Q., Xianghua H., Jingkun Z. Controlled releasing of SDF-1α in chitosan-heparin hydrogel for endometrium injury healing in rat model. Int. J. Biol. Macromol. 2020;143:163–172. doi: 10.1016/j.ijbiomac.2019.11.184. [DOI] [PubMed] [Google Scholar]
- 158.Cai H., Wu B., Li Y., Liu Y., Shi L., Gong L., Xia Y., Heng B.C., Wu H., Ouyang H., Zhu Z., Zou X. Local delivery of silk-cellulose incorporated with stromal cell-derived factor-1α functionally improves the uterus repair. Tissue Eng. 2019;25:1514–1526. doi: 10.1089/ten.TEA.2018.0283. [DOI] [PubMed] [Google Scholar]
- 159.Li X.a., Sun H., Lin N., Hou X., Wang J., Zhou B., Xu P., Xiao Z., Chen B., Dai J., Hu Y. Regeneration of uterine horns in rats by collagen scaffolds loaded with collagen-binding human basic fibroblast growth factor. Biomaterials. 2011;32:8172–8181. doi: 10.1016/j.biomaterials.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 160.Cai Y., Wu F., Yu Y., Liu Y., Shao C., Gu H., Li M., Zhao Y. Porous scaffolds from droplet microfluidics for prevention of intrauterine adhesion. Acta Biomater. 2019;84:222–230. doi: 10.1016/j.actbio.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 161.Xu H.-L., Xu J., Shen B.-X., Zhang S.-S., Jin B.-H., Zhu Q.-Y., ZhuGe D.-L., Wu X.-Q., Xiao J., Zhao Y.-Z. Dual regulations of thermosensitive heparin-poloxamer hydrogel using ε-polylysine: bioadhesivity and controlled KGF release for enhancing wound healing of endometrial injury. ACS Appl. Mater. Interfaces. 2017;9:29580–29594. doi: 10.1021/acsami.7b10211. [DOI] [PubMed] [Google Scholar]
- 162.Lin N., Li X.a., Song T., Wang J., Meng K., Yang J., Hou X., Dai J., Hu Y. The effect of collagen-binding vascular endothelial growth factor on the remodeling of scarred rat uterus following full-thickness injury. Biomaterials. 2012;33:1801–1807. doi: 10.1016/j.biomaterials.2011.11.038. [DOI] [PubMed] [Google Scholar]
- 163.Wang J., Hu R., Xing Q., Feng X., Jiang X., Xu Y., Wei Z. Exosomes derived from umbilical cord mesenchymal stem cells alleviate mifepristone-induced human endometrial stromal cell injury. Stem Cell. Int. 2020;2020 doi: 10.1155/2020/6091269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wang X., Bao H., Liu X., Wang C., Hao C. Effects of endometrial stem cell transplantation combined with estrogen in the repair of endometrial injury. Oncol. Lett. 2018;16:1115–1122. doi: 10.3892/ol.2018.8702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhang S.-S., Xia W.-T., Xu J., Xu H.-L., Lu C.-T., Zhao Y.-Z., Wu X.-Q. Three-dimensional structure micelles of heparin-poloxamer improve the therapeutic effect of 17β-estradiol on endometrial regeneration for intrauterine adhesions in a rat model. Int. J. Nanomed. 2017;12:5643–5657. doi: 10.2147/ijn.S137237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tewary S., Lucas E.S., Fujihara R., Kimani P.K., Polanco A., Brighton P.J., Muter J., Fishwick K.J., Costa M.J.M.D.D., Ewington L.J., Lacey L., Takeda S., Brosens J.J., Quenby S. Impact of sitagliptin on endometrial mesenchymal stem-like progenitor cells: a randomised, double-blind placebo-controlled feasibility trial. EBioMedicine. 2020;51 doi: 10.1016/j.ebiom.2019.102597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mahajan N., Sharma S. The endometrium in assisted reproductive technology: how thin is thin? J. Hum. Reprod. Sci. 2016;9:3–8. doi: 10.4103/0974-1208.178632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Xu F., Shen X., Sun C., Xu X., Wang W., Zheng J. The effect of mitomycin C on reducing endometrial fibrosis for intrauterine adhesion. Med. Sci. Monit. 2020;26 doi: 10.12659/msm.920670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Xu X.-X., Zhang S.-S., Lin H.-L., Lin Q., Shen L.-E., Ansong E., Wu X.-Q. Metformin promotes regeneration of the injured endometrium via inhibition of endoplasmic reticulum stress-induced apoptosis. Reprod. Sci. 2019;26:560–568. doi: 10.1177/1933719118804424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lin J., Wang Z., Huang J., Tang S., Saiding Q., Zhu Q., Cui W. Microenvironment-protected exosome-hydrogel for facilitating endometrial regeneration, fertility restoration, and live birth of offspring. Small. 2021;17 doi: 10.1002/smll.202007235. [DOI] [PubMed] [Google Scholar]
- 171.Liu X., Yang Y., Niu X., Lin Q., Zhao B., Wang Y., Zhu L. An in situ photocrosslinkable platelet rich plasma - complexed hydrogel glue with growth factor controlled release ability to promote cartilage defect repair. Acta Biomater. 2017;62:179–187. doi: 10.1016/j.actbio.2017.05.023. [DOI] [PubMed] [Google Scholar]
- 172.Su T., Huang K., Ma H., Liang H., Dinh P.-U., Chen J., Shen D., Allen T.A., Qiao L., Li Z., Hu S., Cores J., Frame B.N., Young A.T., Yin Q., Liu J., Qian L., Caranasos T.G., Brudno Y., Ligler F.S., Cheng K. Platelet-inspired nanocells for targeted heart repair after ischemia/reperfusion injury. Adv. Funct. Mater. 2019;29 doi: 10.1002/adfm.201803567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Xi J., Cheng J., Jin C.-C., Liu J.-Y., Shen Z.-R., Xia L.-J., Li Q., Shen J., Xia Y.-B., Xu B. Electroacupuncture improves pregnancy outcomes in rats with thin endometrium by promoting the expression of pinopode-related molecules. BioMed Res. Int. 2021;2021 doi: 10.1155/2021/6658321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Jo J., Lee Y.J. Effectiveness of acupuncture in women with polycystic ovarian syndrome undergoing in vitro fertilisation or intracytoplasmic sperm injection: a systematic review and meta-analysis. Acupunct. Med. 2017;35:162–170. doi: 10.1136/acupmed-2016-011163. [DOI] [PubMed] [Google Scholar]
- 175.Manheimer E., Windt D.l. v. d., Cheng K., Stafford K., Liu J., Tierney J., Lao L., Berman B.M., Langenberg P., Bouter L.M. The effects of acupuncture on rates of clinical pregnancy among women undergoing in vitro fertilization: a systematic review and meta-analysis. Hum. Reprod. Update. 2013;19:696–713. doi: 10.1093/humupd/dmt026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Huang X., Chen L., Xia Y.-B., Xie M., Sun Q., Yao B. Effects of electroacupuncture on luteal regression and steroidogenesis in ovarian hyperstimulation syndrome model rat. Life Sci. 2018;197:1–9. doi: 10.1016/j.lfs.2018.01.026. [DOI] [PubMed] [Google Scholar]
- 177.Li C.H., Zhao Y.F., Bo J., Ren X.X., Jiang Z. [Effect of electroacupuncture on the uterine microcirculation in dysmenorrhea rats] Acupunct. Res. 2011;36:12–17. [PubMed] [Google Scholar]
- 178.Ma S., Li D., Feng Y., Jiang J., Shen B. Effects of electroacupuncture on uterine morphology and expression of oestrogen receptors in ovariectomised rats. Acupunct. Med. 2016;35:208–214. doi: 10.1136/acupmed-2016-011093. [DOI] [PubMed] [Google Scholar]
- 179.So E.W.S., Ng E.H.Y. Acupuncture in reproductive medicine. Womens Health. 2010;6:551–563. doi: 10.2217/whe.10.39. [DOI] [PubMed] [Google Scholar]
- 180.Zheng C.H., Huang G.Y., Zhang M.M., Wang W. Effects of acupuncture on pregnancy rates in women undergoing in vitro fertilization: a systematic review and meta-analysis. Fertil. Steril. 2012;97:599–611. doi: 10.1016/j.fertnstert.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 181.Sultan M.T., Choi B.Y., Ajiteru O., Hong D.K., Lee S.M., Kim H.-J., Ryu J.S., Lee J.S., Hong H., Lee Y.J., Lee H., Suh Y.J., Lee O.J., Kim S.H., Suh S.W., Park C.H. Reinforced-hydrogel encapsulated hMSCs towards brain injury treatment by trans-septal approach. Biomaterials. 2021;266 doi: 10.1016/j.biomaterials.2020.120413. [DOI] [PubMed] [Google Scholar]
- 182.Emelyanov A.N., Borisova M.V., Kiryanova V.V. Model acupuncture point: bone marrow-derived stromal stem cells are moved by a weak electromagnetic field. World J. Stem Cell. 2016;8:342–354. doi: 10.4252/wjsc.v8.i10.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Salazar T.E., Richardson M.R., Beli E., Ripsch M.S., George J., Kim Y., Duan Y., Moldovan L., Yan Y., Bhatwadekar A., Jadhav V., Smith J.A., McGorray S., Bertone A.L., Traktuev D.O., March K.L., Colon-Perez L.M., Avin K.G., Sims E., Mund J.A., Case J., Deng X., Kim M.S., McDavitt B., Boulton M.E., Thinschmidt J., Calzi S.L., Fitz S.D., Fuchs R.K., Warden S.J., McKinley T., Shekhar A., Febo M., Johnson P.L., Chang L.-J., Gao Z., Kolonin M.G., Lai S., Ma J., Dong X., White F.A., Xie H., Yoder M.C., Grant M.B. Electroacupuncture promotes central nervous system-dependent release of mesenchymal stem cells. Stem Cell. 2017;35:1303–1315. doi: 10.1002/stem.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Liu T.-M. Application of mesenchymal stem cells derived from human pluripotent stem cells in regenerative medicine, World. J. Stem Cell. 2021;13:1826–1844. doi: 10.4252/wjsc.v13.i12.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Deng J., Zhang Y., Xie Y., Zhang L., Tang P. Cell transplantation for spinal cord injury: tumorigenicity of induced pluripotent stem cell-derived neural stem/progenitor cells. Stem Cell. Int. 2018 doi: 10.1155/2018/5653787. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Poulos J. The limited application of stem cells in medicine: a review. Stem Cell Res. Ther. 2018;9:1. doi: 10.1186/s13287-017-0735-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Worku M.G. Pluripotent and multipotent stem cells and current therapeutic applications: review. Stem Cells Cloning. 2021;14:3–7. doi: 10.2147/sccaa.S304887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zhou L.N., Wang J.C., Zilundu P.L.M., Wang Y.Q., Guo W.P., Zhang S.X., Luo H., Zhou J.H., Deng R.D., Chen D.F. A comparison of the use of adipose-derived and bone marrow-derived stem cells for peripheral nerve regeneration in vitro and in vivo. Stem Cell Res. Ther. 2020;11:153. doi: 10.1186/s13287-020-01661-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kawagishi-Hotta M., Hasegawa S., Igarashi T., Yamada T., Takahashi M., Numata S., Kobayashi T., Iwata Y., Arima M., Yamamoto N., Yagami A., Nakata S., Uzawa T., Matsunaga K., Sugiura K., Akamatsu H. Enhancement of individual differences in proliferation and differentiation potentials of aged human adipose-derived stem cells. Regen. Ther. 2017;6:29–40. doi: 10.1016/j.reth.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Tabatabaei F.S., Torshabi M. In vitro proliferation and osteogenic differentiation of endometrial stem cells and dental pulp stem cells. Cell Tissue Bank. 2017;18:239–247. doi: 10.1007/s10561-017-9620-y. [DOI] [PubMed] [Google Scholar]
- 191.Kou L., Jiang X., Xiao S., Zhao Y.-Z., Yao Q., Chen R. Therapeutic options and drug delivery strategies for the prevention of intrauterine adhesions. J. Contr. Release. 2020;318:25–37. doi: 10.1016/j.jconrel.2019.12.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that supports this study is available from the authors upon reasonable request.