Abstract
Background:
Cardioneuroablation is one of the emerging therapies in vasovagal syncope. In this study, we present a simple method of cardioneuroablation performed via a right-sided approach, targeting anterior-right and right-inferior ganglionated plexi, along with procedural and follow-up data.
Methods:
Patients who had underwent cardioneuroablation between March 2018 and September 2019 with vasovagal syncope in 2 clinics were enrolled in the study. All patients underwent radio-anatomically guided radiofrequency ablation targeting anterior-right ganglionated plexi and right-inferior ganglionated plexi. Syncope and symptom burden, 24-hour ambulatory electrocardiogram data at presentation, and at follow-up were assessed along with procedural data.
Results:
A total of 23 patients underwent modified right-sided cardioneuroablation. Mean basal cycle length decreased significantly from 862.3 ± 174.5 ms at the beginning of the procedure 695.8 ± 152.1 ms following the final radiofrequency ablation (P < .001). Mean 24-hour ambulatory heart rate increased significantly from 66.4 ± 10.7 bpm at baseline to 80 ± 7.6 bpm at follow-up (P < .001). Only 1 patient had 1 episode of syncope following the procedure at the mean follow-up period of 10 ± 2.9 months. The same patient had recurrent presyncope.
Conclusion:
The right-sided cardioneuroablation approach was found to be an effective treatment for vasovagal syncope and may be regarded as a default initial cardioneuroablation technique.
Keywords: Cardioneuroablation, intermittent AV block, radiofrequency ablation, vasovagal syncope
Highlights
Cardioneuroablation is a novel treatment modality for vasovagal syncope.
Initial studies have employed a biatrial cardioneuroablation technique; however, currently, there is no default technique.
In this study group, a limited right-sided cardioneuroablation yielded good symptom-free results in the follow-up.
Introduction
Cardioneuroablation (CNA) is a recently introduced treatment modality for vasovagal syncope (VVS), symptomatic bradycardia, and functional atrioventricular (AV) block, aiming to modify parasympathetic input to the heart by endocardial ablation at sites which have parasympathetic ganglia on the epicardium.1 In canine studies, following 3 main parasympathetic ganglia were defined in, each of which is located in an epicardial fat pad2: (1) junction between medial superior vena cava (SVC) and aortic root, (2) junction between the inferior vena cava (IVC) and left atrium (LA), and (3) junction between right upper pulmonary vein (PV) and LA. Researchers have additionally identified 4 ganglionated plexus (GPs) located in close proximity to the pulmonary veins (anterior right, inferior right, upper left, and inferior left GPs). The initially described technique of CNA requires transseptal puncture for LA access to anatomically ablate all 4 ganglia. Recently, however, intraprocedural heart rate increase during CNA was observed mainly during ablation of right anterior GP, which is anatomically very close to the junction between medial SVC and aortic root.3 While this target is crucial for sinus node autonomic regulation, the inferior right GP may be the main modulator of vagal innervation of the AV node.4 Obviously, these findings raise an important question of whether an effective CNA can be performed with right-only approach, with both anterior right and inferior right GPs targeted by their anatomically close structures in the right atrium (RA). In this study, we report procedural and follow-up data of the study participants who have undergone this modified right-only CNA.
Methods
This was a prospective follow-up study aiming to determine procedural and clinical outcomes in patients who had undergone the modified right-only CNA. Patients were enrolled in the study between March 2018 and September 2019. The procedures were performed, and the patients were clinically followed in 2 tertiary care centers. The study complies with ethical principles defined by Declaration of Helsinki. The study protocol was approved and supervised by the Local Ethical Committee. Written informed consent was obtained from all study participants.
Patient Selection
All patients who had undergone modified right-only CNA for resistant VVS were enrolled in the study. The diagnosis of VVS was based on clinical history and ambulatory electrocardiogram (ECG), and head-up tilt (HUT) testing was not required for inclusion. All patients had documented bradycardia which correlated with their symptoms. Therefore, the patient group consisted of both cardio-inhibitory and mixed-type VVS. Criteria defined previously in 2018 European Society of Cardiology Guidelines for the diagnosis and management of syncope were used for diagnostic confirmation.5 All patients included in the study had recurrent symptoms despite guideline-directed therapy (including counter-pressure maneuvers, tilt training, and when deemed necessary medical therapy with midodrine/fludrocortisone). Exclusion of structural heart disease (i.e. coronary artery disease and heart failure) as well as permanent conduction diseases (permanent AV block and bi-, tri-fascicular block) was required for patients to be included in the study. Patients with documented bradycardia with no symptom correlation were excluded. Patients on midodrine/fludrocortisone therapy prior to CNA were excluded. Finally, patients who underwent ablation of atrial or ventricular arrhythmias during the same session were excluded from the study.
Clinical Data Collection
Pre-procedural clinical data and intraprocedural data were collected during the index procedure. All patients had 24-hour ambulatory ECG before the procedure and during the follow-up visit, approximately 90 days after the procedure. Symptomatic end-point data were collected during the follow-up in following manner: all patients received monthly telephone call and bi-monthly clinic visits. Symptomatic data consisting of syncope and presyncope attributed to VVS were collected. Presyncope was defined as a clinical symptom consistent with VVS without the transient loss of consciousness.
Electrophysiology and Intraprocedural Data Collection
Patients were taken to the cardiac electrophysiology laboratory in a fasting state and the procedures were performed under local anesthesia. During the procedure, fentanyl and/or midazolam was administered, as necessary. Following the local anesthesia, two 8F intravascular sheaths were placed in the femoral vein. All patients received an appropriate dose of unfractionated heparin. A decapolar catheter was placed in the coronary sinus (CS) thereafter. Upon this, His recording was obtained using a quadripolar catheter in all patients, and AH and HV intervals were measured. After the pre-procedural data collection, an irrigated mapping and radiofrequency (Rf) ablation catheter was advanced and 3D electroanatomic map of the RA was created using Carto 3 (Biosense Webster, Diamond Bar, Calif, USA) or Ensite Precision (Abbott, Chicago, Illinois, USA) electroanatomic mapping systems.
Anatomical Targeting and Ablation
The technique of right-sided-only CNA employed in this study had 2 main targets of ablation (Figure 1). First, anterior right GP was targeted using fluoroscopic anatomy and electroanatomic map of RA at the posteroseptal side of SVC-RA junction. Prior to ablation, phrenic nerve capture was tested by pacing at high output (i.e., 25mA). Radiofrequency energy was applied to this site using irrigated tip Rf ablation catheter with power set to 45 W, with temperature and impedance monitoring. Each Rf energy was delivered for minimum 60 seconds and until the loss or ≥80% abatement of local signal. During the ablation, heart rate was actively monitored, and if sinus pause >3 seconds was observed, ventricular pacing was performed. During the ablation, it was expected that basal cycle length (BCL) would increase initially before gradual shortening, which was considered a classical response.
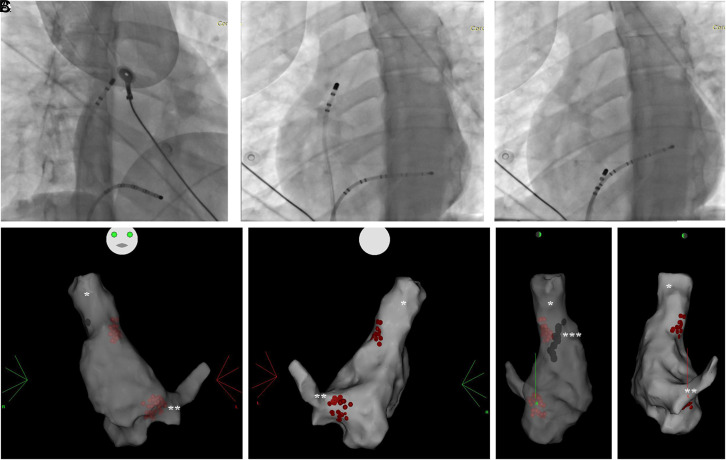
Figure 1.
(A) Right anterior oblique view with decapolar coronary sinus catheter (**) and ablation catheter (*) targeting anterior right (AR) ganglionated plexus (GP) (B). Same catheter position as in A, left anterior oblique view (C). Left anterior oblique view of decapolar coronary sinus catheter(**) and ablation catheter (*) targeting inferior right (IR) GP D-G 3D electroanatomic map right atrium (RA). Radiofrequency lesions (red dots) in right atrium superior vena cava (*) junction and right atrium nferior vena cava coronary sinus (**) junction with right pulmonary veins can be observed. Phrenic capture sites were annotated (***).
The second target of the CNA was the right inferior GP, targeted using fluoroscopic anatomy and electroanatomic map, at the site which is usually located beginning from posterior part of CS ostium until the IVC-RA junction where no atrial signal could be recorded. As the AV node is in close proximity to this region, extreme caution was employed and the AV conduction and any impending damage to this system were always monitored. Radiofrequency energy was delivered with a irrigated tip Rf ablation catheter with similar settings to the first target. After the loss of the local signal, ablation procedure was terminated. Patients were kept in laboratory for another 30 minutes in upon which His recording, AH and HV intervals were re-obtained and then the procedures were terminated. Patients were kept in laboratory for another 30 minutes in upon which His recording, AH and HV intervals were re-obtained and then the procedures were terminated. The 30 minutes waiting period was not included to the procedure time. Increase >20% in heart rate and maintenance of this increase at the end of the waiting period were necessary to classify the procedure as success. All intraprocedural data were collected.
Statistical Analysis
Statistical analyses were performed using a Statistical Package for the Social Sciences software package (version 23.0 for Windows, IBM). Data are expressed as numbers and percentages for discrete variables and as means ± SD for continuous variables. Normal distribution of the variables was assessed with Shapiro–Wilk test. Pre vs post comparisons were made using paired sample t-test and Wilcoxon t-test for parametric and non-parametric variables, respectively. All probabilities were 2-tailed and P < .05 was considered significant.
Results
Twenty-three patients underwent CNA in 2 university centers. The mean age of the study group was 40.7 ± 13.2 years and 10 (43.5%) patients were female. Additional data regarding baseline characteristics of the study group is available on Table 1. All patients had a history of VVS, 3 patients had documented AV block, and 2 patients had documented spontaneous asystolic pause in ambulatory ECG recording. Patients were highly symptomatic with reported mean 3.3 syncope episodes in the year prior to the CNA. Procedural data are available in Table 2. In summary, BCL decreased significantly from 862.3 ± 174.5 to 695.8 ± 152.1 after the procedure. AH interval shortened significantly from 77.0 ± 14.6 to 66.1 ± 13.1, while change in HV interval was not significant (51.2 ± 6.9 vs. 49.8 ± 6.2, prior and after the ablation, respectively). All patients displayed a classical bimodal BCL response during first Rf ablation: initial prolongation of BCL before shortening. In 5 patients, during initial Rf ablation, sinus pause >3 seconds was observed requiring temporary ventricular pacing during the energy delivery, however continued Rf application eventually resulted in an acceleration of the sinus rhythm in all patients (Figure 2). No complications were observed both during the procedure and in the follow-up.
Table 1.
Baseline Characteristics of the Study Group
| Sex, n (%) | |
| Female | 10 (43.5) |
| Male | 13 (56.5) |
| Age (years) | 40.7 ± 13.2 |
| Diabetes mellitus, n (%) | 0 (0) |
| Coronary artery disease, n (%) | 1 (4.3) |
| Left ventricular ejection fraction % | 61.1 ± 5.6 |
| Vasovagal syncope, n (%) | 23 (100) |
| Total number of episodes | 6.2 ± 1.9 |
| Episodes in the last year | 3.3 ± 1.3 |
| Patients who reported presyncope, n (%) | 20 (87) |
| Documented AV block, n (%) | 3 (13) |
| Documented asystolic pause (>3 seconds), n (%) | 2 (8.7) |
Table 2.
Procedural Characteristics of the Cardioneuroablation Procedure
| Procedure time (minutes) | 37.09 ± 22.6 |
| BCL (ms) | |
| Prior to ablation | 862.3 ± 174.5 |
| After the ablation | 695.8 ± 152.1 |
| P | <.001 |
| AH interval (ms) | |
| Prior to ablation | 77.0 ± 14.6 |
| After the ablation | 66.1 ± 13.1 |
| P | .007 |
| HV interval (ms) | |
| Prior to ablation | 51.2 ± 6.9 |
| After the ablation | 49.8 ± 6.2 |
| P | .603 |
| Bimodal BCL response during first radiofrequency ablation, % (n) | 100 (22) |
| Peri-procedural complications | |
| Bleeding complications | 0 |
| Pericardial effusion | 0 |
| Myocardial rupture | 0 |
BCL, basal cycle length.
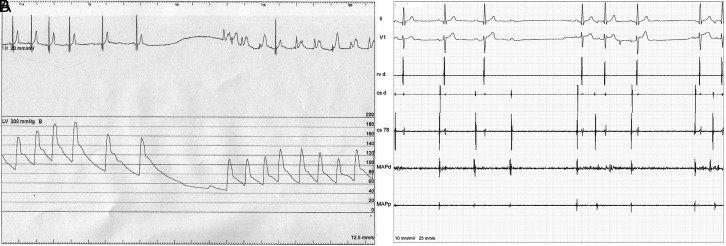
Figure 2.
Pressure (A) and intracardiac electrocardiogram (B) tracings from a patient during modified right-sided cardioneuroablation. During energy delivery, initially, a vagal stimulation is observed manifesting as sinus pause or functional atrioventricular block (B) and sudden drop in blood pressure. When necessary, right ventricular pacing is performed. Both blood pressure and heart rate recover with additional energy delivery.
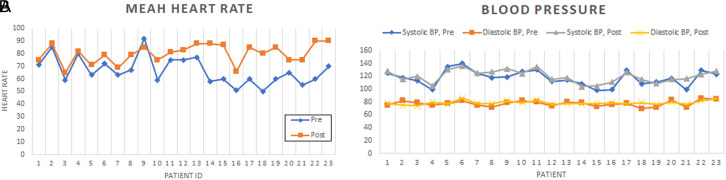
The mean follow-up time was 10 ± 2.9 months, with shortest follow-up of 5 months and the longest of 16 months. The post-procedural ambulatory ECG was performed at mean of 92 days after the procedure (range 87-97). The mean and minimal heart rate in 24-hour ambulatory ECG increased significantly from 66.4 ± 10.7 bpm and 45.7 ± 9.5 bpm to 80 ± 7.6 bpm and 57.4 ± 7.3 bpm, respectively (Table 3). The increase in maximal heart rate was not significant; nevertheless, it displayed a trend for increase from the initial 138.7 ± 22.5 bpm to 144.2 ± 15.9 bpm. Heart rate variability indices decreased significantly signaling profound vagal denervation at the follow-up ambulatory ECG. Figure 3 depicts mean heart rate and blood pressure changes at baseline and at clinical follow-up.
Table 3.
Follow-Up Characteristics of the Study Population
| Follow-up duration (months) | |
| Mean | 10 ± 2.9 |
| Minimum (months) | 5 |
| Maximum (months) | 16 |
| 24 ambulatory ECG heart rate before procedure | |
| Maximum (bpm) | 138.7 ± 22.5 |
| Minimum (bpm) | 45.7 ± 9.5 |
| Mean (bpm) | 66.4 ± 10.7 |
| 24 ambulatory ECG heart rate after the procedure | |
| Maximum (bpm) | 145.4 ± 17.5 |
| Minimum (bpm) | 57.4 ± 7.3 |
| Mean (bpm) | 80 ± 7.6 |
| Pre vs. post ambulatory ECG heart rate recording comparison | |
| Maximum (P) | .110 |
| Minimum (P) | <.001 |
| Mean (P) | <.001 |
| Heart rate variability data, before the procedure | |
| SDNN (ms) | 164 ± 24 |
| Index SDNN (ms) | 68 ± 16 |
| rMSSD (ms) | 39 ± 10 |
| pNN50 (%) | 16 ± 7 |
| Heart rate variability data, after the procedure | |
| SDNN (ms) | 72 ± 18 |
| Index SDNN (ms) | 21 ± 5 |
| rMSSD (ms) | 12 ± 2 |
| pNN50 (%) | 0.8 ± 1 |
| Pre vs. post heart rate variability comparison | |
| SDNN (ms) | <0.001 |
| Index SDNN (ms) | <0.001 |
| rMSSD (ms) | <0.001 |
| pNN50 (%) | <0.001 |
| Blood pressure | |
| Systolic, pre | 117 ± 12 |
| Systolic, post | 120 ± 9 |
| P, systolic | .038 |
| Diastolic, pre | 77 ± 4 |
| Diastolic, post | 78 ± 3 |
| P, diastolic | .196 |
| Atrioventricular block on follow-up ECG | 0 |
| Asystolic pause (>3 seconds) on follow-up | 0 |
| Syncope at follow-up, % (n) | 3.0 (1) |
| Disease-related symptoms at other patients follow-up, % (n) | 0 (0) |
| Medications at follow-up, % (n) | |
| Fludrocortisone | 0 (0) |
| Midodrine | 0 (0) |
ECG, electrocardiogram; SDNN, Standard deviation of NN intervals.
Figure 3.
Mean heart rate on 24-hour ambulatory Holter (A) and blood pressure (B) prior to and after modified right-sided cardioneuroablation. The data are displayed for each patient enrolled.
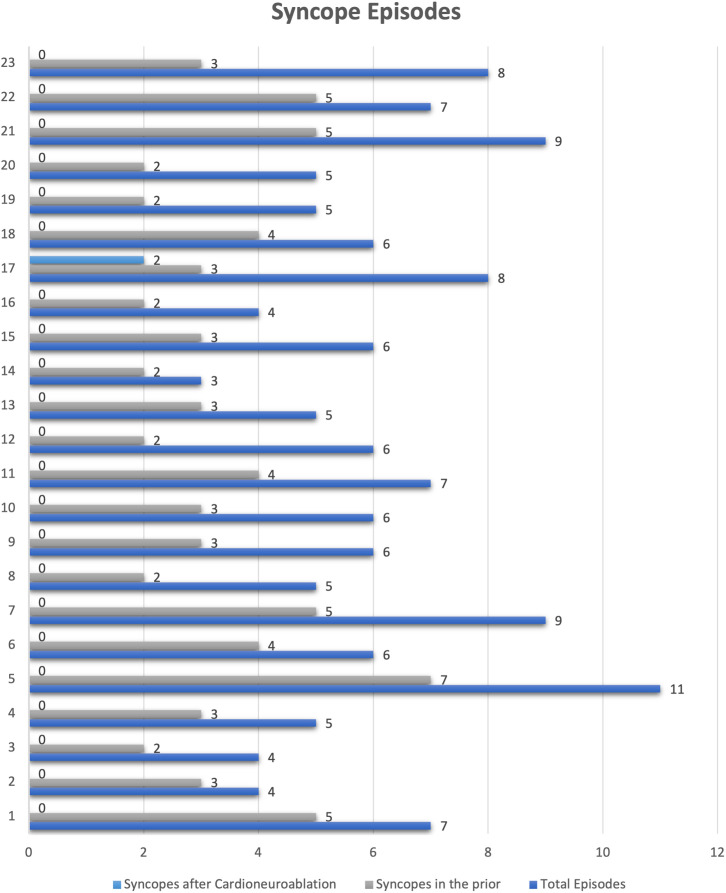
Finally, only 1 patient had syncope recurrence, as well as 2 episodes of presyncope and no patients had documented AV block in the follow-up. (Figure 4) Redo procedure with additional left-sided ablation approach was offered to the patient with recurrent syncope; however, due to overall improvement, she declined. No complications were observed.
Figure 4.
Patient-by-patient syncope episodes.
Discussion
In this study, both intraprocedural and follow-up data indicate very reasonable effectiveness of the procedure among patients who underwent modified right-only CNA for VVS. The data were consistent among study patients, thus suggesting good reproducibility of the technique. All patients enrolled were highly symptomatic at the baseline. After CNA, a dramatic increase in minimal and mean heart rate was observed. Syncope burden had dramatically decreased in the follow-up. Furthermore, while active use of fludrocortisone/midodrine prior to CNA was exclusion criteria, at follow-up, it was permitted; however, none of the study patients necessitated any medical therapy.
Ganglionated Plexus Anatomy and Targeting Rationale, and Mechanisms of Effect
Previous studies of autonomic innervation of the human heart have identified multiple GPs in epicardial fat pads, with the highest density observed posterior to LA and RA.6,7 In vivo, the autonomic innervation can be identified by high-frequency stimulation (HFS), where positive response and therefore site of GP are confirmed when R-R prolongation is observed.8 The GPs around PVs were classified by anatomical location, with right anterior GP located anterior to right superior PV, left upper GP located at the roof of LA, and right and left GPs located inferior to right and left PVs.9 Studies of autonomic modulation have primarily concentrated on the left atrium, as many observations have been made regarding modulation of autonomic response and the relationship between AF and ablation.10 Even though the GPs are abundant epicardially, several evidence point to the fact that localized ‘centers’ for sinus and AV node autonomic innervation may exist. Chiou et al2 have demonstrated that most of the vagal innervation to atria comes from the ‘‘third fat pad’’ located between SVC and the aortic root junction. The junction is anatomically close to the right anterior GP (Figure 1) and 2 of the previous studies have demonstrated that heart rate increase occurs mainly during ablation of these structures.1,3,11 In their study, they have also observed that few vagal fibers may bypass the ‘third fat pad’ and directly project to IVC-LA and right PV-LA GPs.2 Two additional observations have shed some light on the critical role of right anterior GP in autonomic tone modulation. First, in patients who were undergoing AF ablation, the vagal response was significantly lower in the group of patients who underwent right-sided PV ablation before left-sided PVs.12 Similarly, Mori et al13 have observed that ablation of right PVs prior to left PVs prevented excessive vagal response during cryoballon ablation, adding to evidence that right anterior GP, which is injured during right PV ablation, is probably the main regulator of autonomic tone.13 The second target of the technique the inferior right GP, which is thought to mainly modulate the AV node and not the sinus node, should theoretically further augment the vagal denervation.4 This target is therefore of greatest importance in patients displaying vagally mediated AV block (VAVB). Anatomically, the inferior right GP is closely related to IVC-LA GP and IVC-RA junction and is presumed to be opposite to the part of the RA endocardium which is between the posterosuperior wall of CS ostium and IVC-RA junction where no atrial signal can be recorded. Therefore, we have hypothesized that targeting both anterior right and inferior right GPs through their anatomical relation in the RA may accomplish both AV and SA node vagal denervation in the substantial number of patients with a similar success rate compared to extensive and potentially risky left-sided/biatrial approach.
In this study, a profound increase in mean and minimal heart rate was observed after the procedure, which was maintained in the third-month follow-up ambulatory ECG. Similarly, heart rate variability indices decreased significantly at follow-up signaling durable mid-term vagal denervation.14 Cardioneuroablation profoundly changes response to tilt testing, as observed by studies where many of the patients displaying cardioinhibitory response before the procedure start displaying vasodepressor response, albeit without symptom reoccurrence.15 This suggests that (1) multiple mechanisms of VVS are present in a single patient and (2) modification of the dominant mechanism may be sufficient to suppress clinical symptoms.
Comparison with Other Studies of Right-Only CNA
Targets of the ablation in our modified right-only CNA were identified using fluoroscopic and electroanatomic anatomy. Our first target, the SVC-RA junction is anatomically close to the ‘‘third fat pad’’ and right upper GP; therefore, high-power endocardial RF ablation delivered for long duration should be sufficient for persistent damage to this epicardial structure. Some of previous studies have combined anatomically guided ablation with additional physiology-guided parameters such as HFS and spectral mapping for the identification of GPs.1,3,16 However, physiology-guided parameters were not uniformly applied in these studies, since anatomic-only approach has been previously reported, albeit, with slightly different targets and techniques.17,18 It must be noted that during HFS, AF can be induced, thus requiring cardioversion and complicating patient-care.3 Anatomic-only approach will therefore not only shorten the procedure but may also be a safer strategy with lower incidence of side effects, when compared with more complex GP identification. The main disadvantage of the anatomic-only approach may be the necessity of expanded number of RF applications at a single target, as was noted in 2 cases previously reported.17
One basic variation of the right-sided approach for CNA has been previously investigated by Debruyne et al.18,19 In these studies, the investigators have targeted the anterior right GP by delivering Rf energy to the SVC-RA junction. In contrast to these studies, our protocol included an additional target, namely the inferior right GP. Similar to our study, the technique proposed by Debruyne et al18,19 used anatomical targets; however, it should be noted that in contrast to our study, computerized tomographic images were merged to the electroanatomic map. Although this approach may increase the anatomical accuracy during the ablation, radiation, cost, and contrast exposure are clear downsides. Finally, in the previously mentioned study by Hu et al3, greatest increase in heart rate during CNA was reproducibly observed during RA-GP ablation; however, this study is different to our study by (a) left atrial approach and (b) routing ablation of left-sided GPs.3
Study Limitations
A number of limitations of our study must be noted. Firstly, HUT test was not routinely performed; however, since history, physical examination, and ECG have high diagnostic accuracy in syncope diagnosis and the HUT test is non-specific, there is a controversy regarding the role of this test in the evaluation of syncope.20 Furthermore, HUT test is usually not recommended for symptom-control assessment. Therefore, absence of routine HUT test for enrollment or follow-up is a controversial subject. Our study is relatively small (22 patients); however, previous studies (i.e. Debruyne et al18,19) are in similar size, probably due to relative low incidence of resistant VVS patients. Next, although all patients were followed with 24-hour ambulatory Holter, internal loop recorder was not available due to insurance and reimbursement issues. This study, due to its design, inherits all limitations of observational studies, and additionally, symptoms were reported by the patients, which obviously is always subject to a placebo effect. To eliminate this possibility, a randomized study is necessary. Of note, in this relatively small 2-center study, data from all patients who had undergone CNA is reported, and no patient was lost to follow-up. A prospective control group undergoing biatrial CNA could have been included; yet, although small, the risks of transseptal puncture and left atrial ablation were considered unnecessary, given that unilateral approach yielded quite favorable outcomes.18,19 Furthermore, relative scarcity of this patient population precluded including a control group within reasonable study time. Finally, although the follow-up period is similar to previous studies, longer follow-up is probably necessary, due to possible axon regeneration and attenuation of the damage to the GP neuron bodies. Nevertheless, despite the possibility of regeneration, even moderate amount of damage may attenuate exaggerated reflexes and bring them to physiological levels.
Conclusion
The data from this study suggest that the modified right-only approach for CNA may be an effective treatment for VVS, VAVB, and sinus bradycardia and is a good candidate for the default CNA technique, reserving more extensive approach (i.e. left-sided) for the few resistant/redo procedures.
Footnotes
Ethics Committee Approval: Ethical committee approval was received from the Ethics Committee of Ankara University (approval No: 5343232).
Informed Consent: Written informed consent was obtained from all participants who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – B.C., E.B., O.B.; Data analysis – B.C., E.B., O.B.; Statistics – N.S., T.S.T., I.M.A.; Drafting – T.A.; Critical revision – K.E., V.K., E.T.
Declaration of Interests: The authors declare that they have no competing interest.
Funding: This study received no funding.
References
- 1. Pachon JC, Pachon EI, Pachon JC.et al. “Cardioneuroablation” – new treatment for neurocardiogenic syncope, functional AV block and sinus dysfunction using catheter RF-ablation. Europace. 2005;7(1):1–13. [DOI] [PubMed] [Google Scholar]
- 2. Chiou CW, Eble JN, Zipes DP. Efferent vagal innervation of the canine atria and sinus and atrioventricular nodes. The third fat pad. Circulation. 1997;95(11):2573 2584. 10.1161/01.cir.95.11.2573) [DOI] [PubMed] [Google Scholar]
- 3. Hu F, Zheng L, Liang E.et al. Right anterior ganglionated plexus: the primary target of cardioneuroablation? Heart Rhythm. 2019;16(10):1545 1551. 10.1016/j.hrthm.2019.07.018) [DOI] [PubMed] [Google Scholar]
- 4. Hou Y, Scherlag BJ, Lin J.et al. Ganglionated plexi modulate extrinsic cardiac autonomic nerve input: effects on sinus rate, atrioventricular conduction, refractoriness, and inducibility of atrial fibrillation. J Am Coll Cardiol. 2007;50(1):61 68. 10.1016/j.jacc.2007.02.066) [DOI] [PubMed] [Google Scholar]
- 5. Brignole M, Moya A, de Lange FJ.et al. 2018 ESC Guidelines for the diagnosis and management of syncope. Eur Heart J. 2018;39(21):1883 1948. 10.1093/eurheartj/ehy037) [DOI] [PubMed] [Google Scholar]
- 6. Pauza DH, Skripka V, Pauziene N, Stropus R. Morphology, distribution, and variability of the epicardiac neural ganglionated subplexuses in the human heart. Anat Rec. 2000;259(4):353 382. [DOI] [PubMed] [Google Scholar]
- 7. Armour JA, Murphy DA, Yuan BX, MacDonald S, Hopkins DA. Gross and microscopic anatomy of the human intrinsic cardiac nervous system. Anat Rec. 1997;247(2):289 298. [DOI] [PubMed] [Google Scholar]
- 8. PO SS, NAKAGAWA H, JACKMAN WM. Localization of left atrial ganglionated plexi in patients with atrial fibrillation. J Cardiovasc Electrophysiol. 2009;20(10):1186 1189. 10.1111/j.1540-8167.2009.01515.x) [DOI] [PubMed] [Google Scholar]
- 9. Stavrakis S, Po S. Ganglionated plexi ablation: physiology and clinical applications. Arrhythm Electrophysiol Rev. 2017;6(4):186-190. 10.15420/aer2017.26.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhao QY, Huang H, Zhang SD.et al. Atrial autonomic innervation remodelling and atrial fibrillation inducibility after epicardial ganglionic plexi ablation. Europace. 2010;12(6):805 810. 10.1093/europace/euq089) [DOI] [PubMed] [Google Scholar]
- 11. Qin M, Zhang Y, Liu X, Jiang WF, Wu SH, Po S. Atrial ganglionated plexus modification: a novel approach to treat symptomatic sinus bradycardia. JACC Clin Electrophysiol. 2017;3(9):950 959. 10.1016/j.jacep.2017.01.022) [DOI] [PubMed] [Google Scholar]
- 12. Hu F, Zheng L, Liu S.et al. Avoidance of vagal response during circumferential pulmonary vein isolation: effect of initiating isolation from right anterior ganglionated plexi. Circ Arrhythm Electrophysiol. 2019;12(12):e007811. 10.1161/CIRCEP.119.007811) [DOI] [PubMed] [Google Scholar]
- 13. Mori H, Kato R, Ikeda Y.et al. Analysis of the heart rate variability during cryoballoon ablation of atrial fibrillation. Europace. 2018;20(8):1259 1267. 10.1093/europace/eux225) [DOI] [PubMed] [Google Scholar]
- 14. Pachon M JC, Pachon-M EI, Pachon CTC.et al. Long-Term Evaluation of the Vagal Denervation by Cardioneuroablation Using Holter and Heart Rate Variability. Circ Arrhythm Electrophysiol. 2020;13(12):e008703. 10.1161/CIRCEP.120.008703) [DOI] [PubMed] [Google Scholar]
- 15. Piotrowski R, Żuk A, Baran J, Sikorska A, Kryński T, Kułakowski P. Cardioneuroablation changes the type of vasovagal response in patients with asystolic reflex syncope. Auton Neurosci. 2021;235:102838. 10.1016/j.autneu.2021.102838) [DOI] [PubMed] [Google Scholar]
- 16. Yao Y, Shi R, Wong T.et al. Endocardial autonomic denervation of the left atrium to treat vasovagal syncope: an early experience in humans. Circ Arrhythm Electrophysiol. 2012;5(2):279 286. 10.1161/CIRCEP.111.966465) [DOI] [PubMed] [Google Scholar]
- 17. Rebecchi M, de Ruvo E, Strano S.et al. Ganglionated plexi ablation in right atrium to treat cardioinhibitory neurocardiogenic syncope. J Interv Card Electrophysiol. 2012;34(3):231 235. 10.1007/s10840-012-9666-5) [DOI] [PubMed] [Google Scholar]
- 18. Debruyne P, Rossenbacker T, Collienne C.et al. Unifocal right-sided ablation treatment for neurally mediated syncope and functional sinus node dysfunction Under computed tomographic guidance. Circ Arrhythm Electrophysiol. 2018;11(9):e006604. 10.1161/CIRCEP.118.006604) [DOI] [PubMed] [Google Scholar]
- 19. Debruyne P, Wijns W. Cardio-neuromodulation: The Right-Sided Approach. JACC Clin Electrophysiol. 2017;3(9):1056 1057. 10.1016/j.jacep.2016.12.027) [DOI] [PubMed] [Google Scholar]
- 20. Kulkarni N, Mody P, Levine BD. Abolish the tilt table test for the workup of syncope! Circulation. 2020;141(5):335 337. 10.1161/CIRCULATIONAHA.119.043259) [DOI] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a