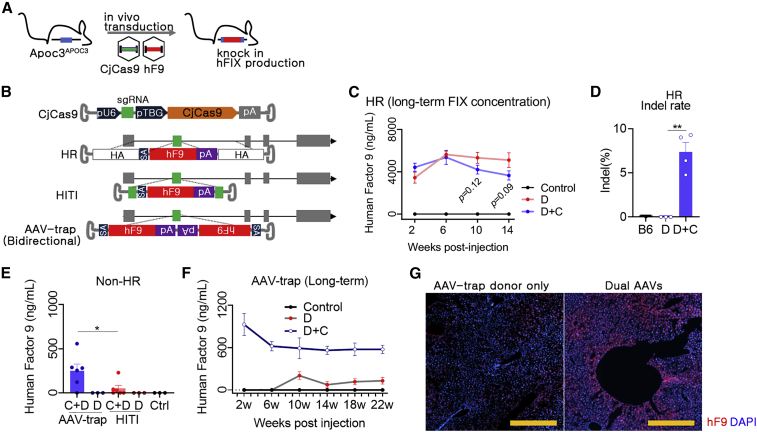
Figure 4.
Expression of therapeutic proteins via HR, HITI, and AAV-trap approaches
(A) Strategy for therapeutic gene knock-in. Dual AAVs were transduced into the humanized mice, and expression by hF9 gene knock-in was confirmed. (B) AAV vector maps for in vivo KI used in this study. AAV-CjCas9 was designed with a U6 promoter, sgRNA, TBG promoter, CjCas9, and polyadenylation signal (pA). Next, three donor templates for HR, HITI, and bidirectional were designed. HA, homology arm, SA; En2SA splicing acceptor; gray box, exon. (C) HR-mediated in vivo expression of hFIX. Control, AAV-untreated mice (n = 4); D, AAV-HR donor only (n = 5); D + C, dual AAVs (AAV-CjCas9 + AAV-HR donor) (n = 6). AAV-HR donor only (2 × 1013 vg/kg) or dual AAVs (2 × 1013 vg/kg for each AAV) were injected intravenously. Then, blood was repeatedly sampled, and hFIX concentrations were analyzed. (D) Indel frequency at the APOC3 target site in AAV-injected liver. (E) KI-mediated expression of hFIX using a non-HR strategy was analyzed. Blood samples were collected 6 weeks after injection of 2 × 1013 vg/kg of each AAV. D, AAV donor only (AAV-trap or HITI); D + C, dual AAVs. Each dot indicates data from an individual mouse. Statistical analysis was performed using the Student t test. ∗p < 0.05, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001. (F) Analysis of long-term effects by AAV-trap strategy. Control, AAV-untreated mice (n = 3); D, AAV-trap donor only (n = 3); D + C, dual AAVs (AAV-CjCas9 + AAV-trap donor) (n = 4). (G) hFIX protein expression by AAV-trap strategy was detected by immunofluorescence analysis of liver tissues. A representative image is shown.