Abstract
The clinical manifestations of SARS-CoV-2 infection mainly involve the respiratory system. However, there is increasing evidence that this virus can affect other organs, causing a wide range of clinical symptoms. This is the report of a 40-day-old patient who presented with sepsis and had no risk factors other than SARS-CoV-2 infection, whose radiological findings were compatible with cerebral sinus vein thrombosis.
Keywords: COVID-19, Thrombosis, Neurological symptom, Coagulopathy
Abbreviations: SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; MIS-C, Pediatric multisystem inflammatory syndrome; COVID-19, Coronavirus disease 2019; RT-PCR, Reverse transcriptase - polymerase chain reaction; CSF, Cerebrospinal fluid; CT, Computed tomography; LMWH, Low-molecular-weight heparin; HIV, Human Immunodeficiency Virus; MTHFR, Metilen Tetra Hidro Folat Redüktaz; GBS, Guillain-Barré syndrome; UFH, Unfractionated heparin
Introduction
In December 2019, the first severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection was reported, and the World Health Organization declared this disease (the novel coronavirus disease 2019, COVID-19) a global pandemic in March 2020 [1]. While SARS-CoV-2 infection generally causes a milder course of the disease in children than in adults, it may also lead to pediatric multisystem inflammatory syndrome (MIS-C), severe cases, and even mortalities [2]. As the pandemic progresses, in addition to respiratory symptoms, other system involvements including the nervous system are being defined [3]. Although severe neurological symptoms are rare in children diagnosed with COVID-19, cerebral sinus vein thrombosis cases have been reported in the literature [3,4,5]. We report an infantile case who was admitted with the clinical sign of sepsis, in whom cerebral sinus vein thrombosis was observed. No major cause could be found in the etiology, and the SARS-CoV-2 reverse transcriptase - polymerase chain reaction (RT-PCR) test of the patient was positive.
Case
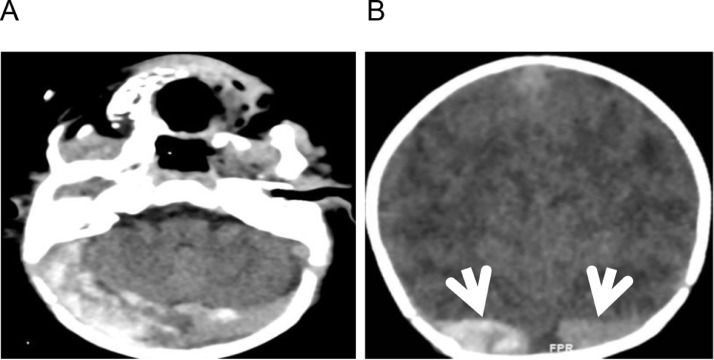
The 40-day-old male patient who was born at week 375/7 of gestation at a weight of 2945 g through spontaneous vaginal delivery was brought to the emergency service with complaints of restlessness, not being able to feed, and sleepiness lasting for 1 day. In his first physical examination at the emergency service, the infant was lethargic, his fontanelle was sunken, his skin turgor was reduced, and he appeared to be dehydrated. The patient who had deteriorated consciousness and circulation had a peak heart rate (PHR) of 182/min, respiratory rate (RR) of 50/min, arterial blood pressure (ABP) of 80/49 mmHg, oxygen saturation (SpO2) of 95%, capillary refill time (CRT) of >3 s, body temperature of 37.9 °C, and weak peripheral pulse. The blood gas test results of the patient were determined as pH: 6.62, PCO2: 29.5 mmHg, HCO3: 3 mmol/L, and lac: 8.8 mmol/L. Normal saline was provided at a dose of 20 cc/kg, and empirical antibiotic treatment was started. The patient in whom respiratory stabilization was achieved by intubation was transferred to the pediatric intensive care unit. His-laboratory tests revealed leukocytosis (86,000/mm3), thrombocytosis (900,000/mm3), anemia (9.2 mg/dL), urea (35 mg/dL), creatine (1.06 mg/dL), uric acid (10.2 mg/dL), and sodium (150 mmol/L). With the values of segment: 68%, stab: 12%, lymphocyte: 16%, and monocyte: 4%, his peripheral smear result was interpreted as a leukemoid reaction. The patient's inflammatory markers (C-reactive protein: 74 mg/dL, procalcitonin: 3.73 ng/mL), cardiac parameters (NT-ProBNP: 5699 pg/mL, troponin: 31.7 pg/mL, Ck-MB: 5.65 ng/mL), d-dimer: 1146 ng/mL and ferritin: 1006 ng/mL were found to be elevated. The patient's thoracic radiography results were normal. Appropriate intravenous fluid and empirical antibiotic treatment was continued in the pediatric intensive care unit. The patient, who did not display a respiratory pathology, was extubated. The patient who had a lethargic appearance did not have a sign of a focal neurological deficit or meningeal irritation. Lumbar puncture was performed to exclude meningitis. No cells were detected in the analysis of the cerebrospinal fluid (CSF). The patient's CSF glucose value was 70 mg/dL (simultaneous blood sugar: 97 mg/dL), his protein value was 65 mg/dL, and his CSF SARS-CoV-2 reverse transcriptase - polymerase chain reaction (RT-PCR) test came out negative. His-CSF culture and meningitis panel results were determined to be normal. His-emergency computed tomography (CT) revealed signs indicating sinus thrombosis in the right transverse sinus and superior sagittal sinus posterior parts (Fig. 1 ), cranial magnetic resonance venography showed acute dural sinus thrombosis involving the posterior part of the superior sagittal sinus and the right transverse and sigmoid sinuses and extending towards the proximal of the left transverse sinus (Figs. 2 , 3 ). Low-molecular-weight heparin (LMWH) (subcutaneous) treatment was started. The patient was examined by Doppler ultrasonography for diffuse thrombus, and an echogenic partial thrombus appearance was observed inside the bilateral jugular vein. It was considered that this could be related to the central venous catheter interventions. In the femoral vein in the catheter-attached right leg, diffuse edema and circulatory impairment developed within hours. Due to the presence of the catheter, Doppler ultrasonography examination could not be made. The patient's clinical status was compatible with thrombus. The LMWH treatment of the patient was continued. With elevation and massage, the clinical symptoms of the patient were reversed in a short time. The patient was examined by echocardiography, and his result was interpreted as a decrease in left ventricular systolic functions. It was thought that this could be secondary to the circulatory impairment, shock status, and deep metabolic acidosis experienced by the patient. Meanwhile, the nasopharyngeal swab SARS-CoV-2 RT-PCR test of the patient for etiology was found positive. A sample from the mother was also sent to the laboratory, and it came out negative. There was no known history of contact with a COVID-19 patient in the anamnesis of the patient.
Fig. 1.
Axial (A) and coronal cross-section (B) non-contrast CT scans: broadened and excessively dense appearance of the left and right transverse sinuses (arrows) compatible with thrombosis.
Fig. 2.
T1-weighted contrast sagittal (A) and axial (B) MR images, superior sagittal and right transverse sinus thrombosis (arrows) appearing as filling defects.
Fig. 3.
Three-dimensional phase-contrast MR angiography MIP images; occlusion of the right transverse sinus and jugular vein due to thrombosis and filling defect in distal part of superior sagittal sinus.
With the clinical picture of shock, cardiac dysfunction, neurological involvement, acute kidney injury, elevated inflammation parameters, and positive test results for SARS-CoV-2 infection, the patient in whom MIS-C could not be excluded was given intravenous immunoglobulin (IVIG) by 2 g/kg with a single dose and started on methylprednisolone at 2 mg/kg/day.
There was no reproduction in the blood, urine, or CSF cultures of the patient, and his serology on the cytomegalovirus, Epstein-Barr virus, Hepatitis B, C viruses, and HIV came out negative. A thrombophilia panel was requested for the patient who did not have a family history of thrombosis. In the genetic thrombophilia panel results of the patient who had multiple thromboses, MTHFR gene mutation, Factor V Leiden mutation, prothrombin gene mutation, anticardiolipin IgM, and IgG tests were negative. His-lupus anticoagulant and lipoprotein (a) results were normal. The patient's Protein C: 32% (N: 32–54%) and Protein S: 49.4% (N: 48–78%) activities were at the lower limit based on his age, whereas his antithrombin III: 52% (N: 63–93%) activity was low. Due to consumption in the acute period of thrombosis, the levels of natural anticoagulants may temporarily decrease in processes like DIC and sepsis, and this symptom needs to be interpreted carefully. The patient's Protein C, Protein S, and antithrombin III activities were checked three months after the treatment, and his results were determined to be normal.
Without a neurological deficit and with subcutaneous LMWH, the patient was hospitalized at the pediatric intensive care unit for 3 days and the pediatric infectious diseases inpatient clinic for 26 days. The LMWH treatment continued for four months. In the patient's follow-up cranial magnetic resonance venography, it was observed that the sinus vein thrombosis had completely regressed, and the results of the neurological and developmental examination of the patient were normal for his age. We are planning to provide LMWH treatment at the prophylaxis dose for three months.
Discussion
SARS-CoV-2 has a broad range of neurological involvement. However, its mechanism has not been completely understood yet. Four potential mechanisms are considered: 1) direct neurotropic effect (e.g., anosmia), 2) systemic inflammatory response (e.g., encephalopathy), 3) immune-mediated para-infectious or post-infectious effect (e.g., GBS), and 4) coagulopathic effects [6]. Coagulopathy is a significant and well-known characteristic of SARS-CoV-2 infection. It may lead to venous thromboembolic events [7]. The interaction between SARS-CoV-2 and endothelial cell surfaces is the main factor that triggers immunothrombosis [8]. Increased systemic inflammation that emerges with the binding of the virus to the angiotensin-2 receptors of endothelial cells and endothelial damage leads to prothrombotic endothelial dysfunction [1]. The role of the ACE-2 receptors, platelet dysfunction, hypoxemia-triggered factors, complement activation, cytokine storms, and the formation of antiphospholipid antibodies are other factors that contribute to SARS-CoV-2-associated hypercoagulopathy/prothrombotic status [9]. Moreover, sepsis-related dehydration, immobilization-related stasis, and direct neuro-invasion may be effective in the etiology of thrombosis [7].
In a meta-analysis, among adults hospitalized due to COVID-19, the general incidence of venous thromboembolism was found as 21%, while this rate was determined as 31% in those who were admitted to the intensive care unit [1]. While thrombotic complications have been reported in pediatric COVID-19 patients, there is a limited knowledge base about their prevalence and risk factors [10,11]. Aguilera-Alonso et al. reported that four patients among 537 pediatric patients infected with SARS-CoV-2 and 1.1% of those who were hospitalized developed thrombotic complications, and cerebral vein thrombosis was identified in one patient [10]. Whitworth et al. determined thrombotic events in 20 of 814 pediatric patients. Thrombotic complications were observed at rates of 6.5% in patients with MIS-C, 2.1% in COVID-19 patients, and 0.7% in asymptomatic patients infected with SARS-CoV-2. In a COVID-19 patient who had an underlying disease, cerebral vein thrombosis was reported. As seen here, the incidence of pediatric thrombotic events has increased with COVID-19, but it is still not as high as the rate seen in hospitalized adults [12].
In a systematic review examining 57 adult patients, while cerebral vein thrombosis was seen at a rate of 0.08% in those hospitalized due to COVID-19, it constituted 4.2% of all cerebrovascular events. According to information obtained from data on adults, while cerebral vein thrombosis is rare in SARS-CoV-2 infection, its risk is relatively increased [13,14].
Pediatric cerebral sinovenous thrombosis is a rarely encountered disease. Its risk is typically multifactorial. Among previously healthy children, the most frequently encountered risk factors are head and neck infections, acute illness, dehydration, and iron deficiency [15]. Our patient had symptoms compatible with dehydration. It is thought that dehydration is involved in the etiology of cerebral sinus vein thrombosis accompanying SARS-CoV-2 infection.
Few cases of cerebral sinus vein thrombosis associated with pediatric SARS-CoV-2 infection have been reported in the literature. In an international study with the collaboration of 61 centers in 26 countries, 33 of 54 pediatric patients with cerebral sinus vein thrombosis were screened for SARS-CoV-2 infection, and 1 (3%) was determined to be positive [16]. A study where 38 pediatric patients infected with SARS-CoV-2 who had neurological involvement were examined, cerebral vein thrombosis was observed in 1 patient (13%) [3].
In treatment, LMWH or unfractionated heparin (UFH) are anticoagulants that are preferred in children. LMWH is more prevalently used compared to UFH. LMWH may also have additional benefits due to its anti-inflammatory and immunomodulatory properties. LMWH also interacts with the SARS-CoV-2 Spike S1 protein receptor binding domain and prevents receptor binding. It was demonstrated that it has a potential antiviral activity for this reason [11]. Considering treatments applied in pediatric patients in the literature, a patient who had tuberculous meningitis and SARS-CoV-2 infection received anti-tuberculosis and aspirin treatments and was discharged with residual left hemiparesis [5]. Another patient was discharged without any sequela following lopinavir-ritonavir, hydroxychloroquine, azithromycin, and unfractionated heparin treatments [4]. In our patient, considering his neurological involvement, cardiac dysfunction, acute kidney injury, elevated acute phase reactants, and positive SARS-CoV-2 result together, a distinction between MIS-C and COVID-19 could not be made. As there are no specific case definitions and confirmatory laboratory tests for MIS-C, it was difficult to distinguish it from COVID-19. Considering the age of the patient, the fact that he did not have respiratory system involvement and the highly limited nature of observational data about the safety or effectiveness of medication treatment in pediatric COVID-19 patients, COVID-19 treatment was not provided to our patient [17]. With the diagnosis of MIS-C, the patient was treated with IVIG and methylprednisolone. It is believed that, with increased numbers of pediatric cases in the COVID-19 pandemic period, protocols for the diagnosis, follow-up, and treatment of the disease will be developed.
Conclusions
Regardless of whether the classical symptoms of COVID-19 are observed in children, case reports on neurological symptoms and SARS-CoV-2 are being published in increasing numbers. Clinicians should include SARS-CoV-2 in the differential diagnosis process for children presenting with new-onset neurological symptoms. Cerebral vein thrombosis should be kept in mind for all pediatric SARS-CoV-2-positive patients who have neurological symptoms.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Malas M.B., Naazie I.N., Elsayed N., Mathlouthi A., Marmor R., Clary B. Thromboembolism risk of COVID-19 is high and associated with a higher risk of mortality: a systematic review and meta-analysis. EClinicalMedicine. 2020;29 doi: 10.1016/j.eclinm.2020.100639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kosmeri C., Koumpis E., Tsabouri S., Siomou E., Makis A. Hematological manifestations of SARS-CoV-2 in children. Pediatr. Blood Cancer. 2020;67(12):e28745. doi: 10.1002/pbc.28745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindan C.E., Mankad K., Ram D., et al. Neuroimaging manifestations in children with SARS-CoV-2 infection: a multinational, multicentre collaborative study. Lancet Child Adolesc. Health. 2021;5(3):167–177. doi: 10.1016/S2352-4642(20)30362-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ippolito Bastidas H., Márquez-Pérez T., García-Salido A., et al. Cerebral venous sinus thrombosis in a pediatric patient with COVID-19. Neurol. Clin. Pract. 2021;11(2):e208–e210. doi: 10.1212/CPJ.0000000000000899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Essajee F., Solomons R., Goussard P., Van Toorn R. Child with tuberculous meningitis and COVID-19 coinfection complicated by extensive cerebral sinus venous thrombosis. BMJ Case Rep. 2020;13(9) doi: 10.1136/bcr-2020-238597. Published 2020 Sep 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silvestri P., Clemente A., Spalice A., et al. Case report: cerebral venous sinus thrombosis in a young child with SARS-CoV-2 infection: the Italian experience. Front. Neurol. 2022;13 doi: 10.3389/fneur.2022.861345. Published 2022 Mar 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mowla A., Shakibajahromi B., Shahjouei S., et al. Cerebral venous sinus thrombosis associated with SARS-CoV-2; a multinational case series. J. Neurol. Sci. 2020;419 doi: 10.1016/j.jns.2020.117183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guendouz C., Quenardelle V., Riou-Comte N., et al. Pathogeny of cerebral venous thrombosis in SARS-Cov-2 infection: case reports. Medicine (Baltimore) 2021;100(10):e24708. doi: 10.1097/MD.0000000000024708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghosh R., Roy D., Mandal A., et al. Cerebral venous thrombosis in COVID-19. Diabetes Metab. Syndr. 2021;15(3):1039–1045. doi: 10.1016/j.dsx.2021.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aguilera-Alonso D., Murias S., Martínez-de-Azagra Garde A., et al. Prevalence of thrombotic complications in children with SARS-CoV-2. Arch. Dis. Child. 2021;106(11):1129–1132. doi: 10.1136/archdischild-2020-321351. [DOI] [PubMed] [Google Scholar]
- 11.Sharathkumar A.A., Faustino E.V.S., Takemoto C.M. How we approach thrombosis risk in children with COVID-19 infection and MIS-C. Pediatr. Blood Cancer. 2021;68(7):e29049. doi: 10.1002/pbc.29049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whitworth H., Sartain S.E., Kumar R., et al. Rate of thrombosis in children and adolescents hospitalized with COVID-19 or MIS-C. Blood. 2021;138(2):190–198. doi: 10.1182/blood.2020010218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baldini T., Asioli G.M., Romoli M., et al. Cerebral venous thrombosis and severe acute respiratory syndrome coronavirus-2 infection: a systematic review and meta-analysis. Eur. J. Neurol. 2022 doi: 10.1111/ene.14727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Mufti F., Amuluru K., Sahni R., et al. Cerebral Venous Thrombosis in COVID-19: a New York Metropolitan Cohort Study. AJNR Am. J. Neuroradiol. 2021;42(7):1196–1200. doi: 10.3174/ajnr.A7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.R Ichord, Cerebral sinovenous thrombosis, Front. Pediatr. (2017). [DOI] [PMC free article] [PubMed]
- 16.Beslow L.A., Linds A.B., Fox C.K., et al. Pediatric ischemic stroke: an infrequent complication of SARS-CoV-2. Ann. Neurol. 2021;89(4):657–665. doi: 10.1002/ana.25991. [DOI] [PubMed] [Google Scholar]
- 17.National Instutites of Health COVID-19 treatment guadlines. Special in Considerations Children. 2021 https://www.covid19treatmentguidelines.nih.gov/special-populations/children/ [Google Scholar]