Figure 3.
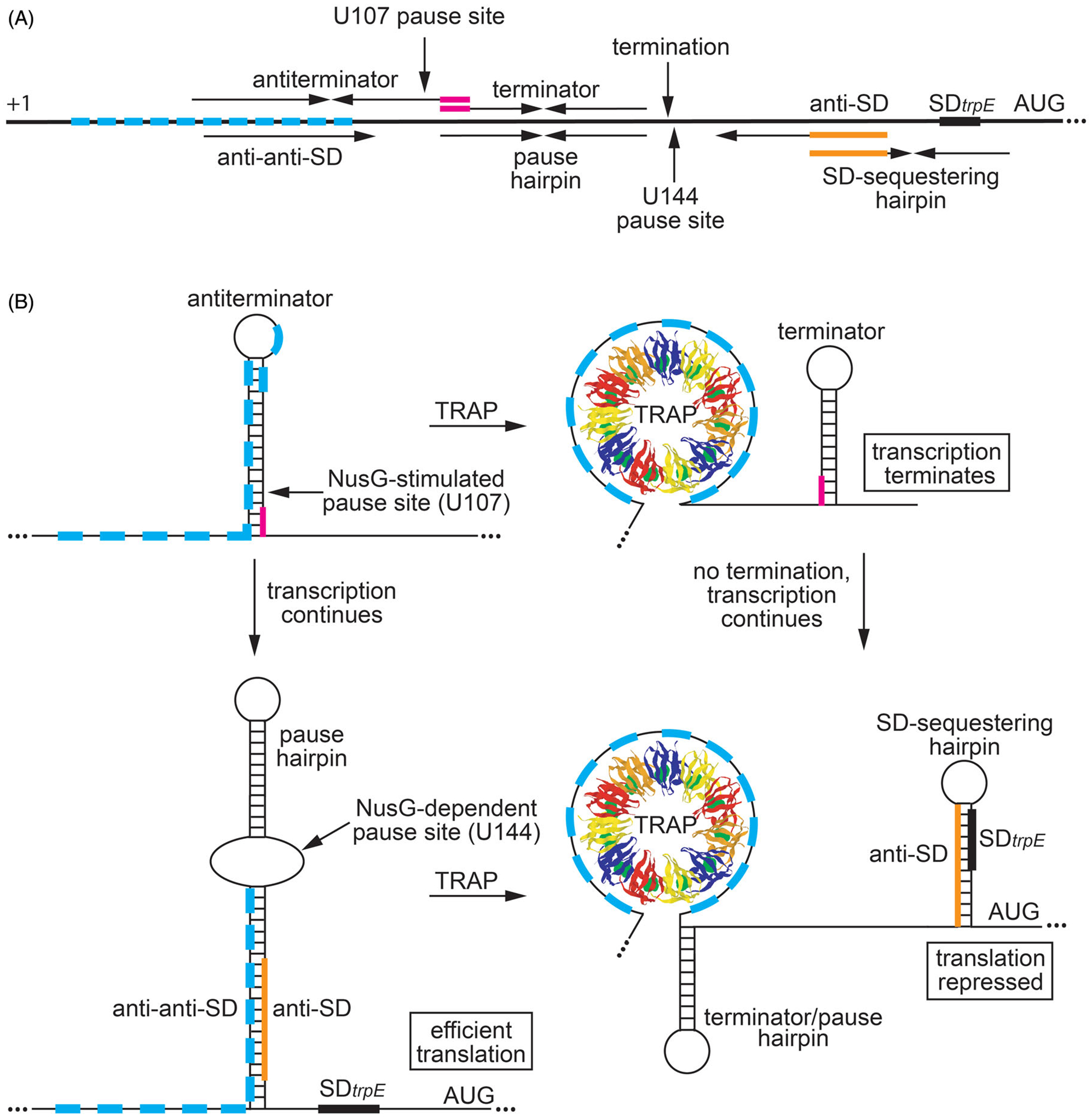
Regulation of trpEDCFBA operon in response to tryptophan availability by transcription attenuation and translational control mechanisms. (A) Schematic representation of the trpEDCFBA operon leader region. The thick black line represents the leader region between the start of transcription (+1) and the AUG start codon for trpE. The 11 trinucleotide repeats within the TRAP binding are shown as cyan boxes. (Top) Components involved in transcription attenuation. Inverted repeats for the overlapping antiterminator and terminator structures (3-nt overlap in magenta), the U107 pause site, and the point of termination (G140) are labeled. (Bottom) Components involved in translation control. The pause hairpin and U144 pause site are labeled. Inverted repeats for the large secondary structure and the overlapping SD-sequestering hairpin are shown (overlap in orange). The anti-anti-SD, anti-SD (orange), and SD sequences are also labeled. (B) Transcription attenuation model (top). During transcription, RNAP pauses at the NusG-dependent pause site at position U107. Under tryptophan-limiting conditions, TRAP does not bind to trp leader RNA, and RNAP eventually overcomes the pause and transcription resumes. In this case, formation of the antiterminator structure prevents the formation of the terminator hairpin, resulting in transcription readthrough into the trp operon structural genes. Under tryptophan-excess conditions, tryptophan-activated TRAP binds to the 11 trinucleotide repeats while RNAP is paused at U107, which prevents the formation of the antiterminator structure. As a consequence, the terminator hairpin forms and transcription terminates at G140. Thus, NusG-stimulated pausing at U107 provides additional time for TRAP to bind and promote termination. trpE translation control model (bottom). During transcription of trp operon readthrough transcripts, RNAP pauses at the NusG-dependent pause site at position U144, which provides a second opportunity for TRAP to bind to the nascent trp transcript. Under tryptophan-limiting conditions, TRAP does not bind to the nascent trp leader transcript, and RNAP eventually overcomes the pause and resumes transcription. In this case, the RNA adopts a structure such that the trpE SD sequence is single-stranded, resulting in efficient translation. Under tryptophan-excess conditions, TRAP can bind to the paused transcript. RNAP eventually overcomes the pause and resumes transcription, which leads to the formation of the trpE SD sequestering hairpin and repression of translation. Note that the same structure functions as the terminator and U144 pause hairpin. Color coding is the same as in (A).
