Fig. 1. Functional characterization and overall structures of human PORCN.
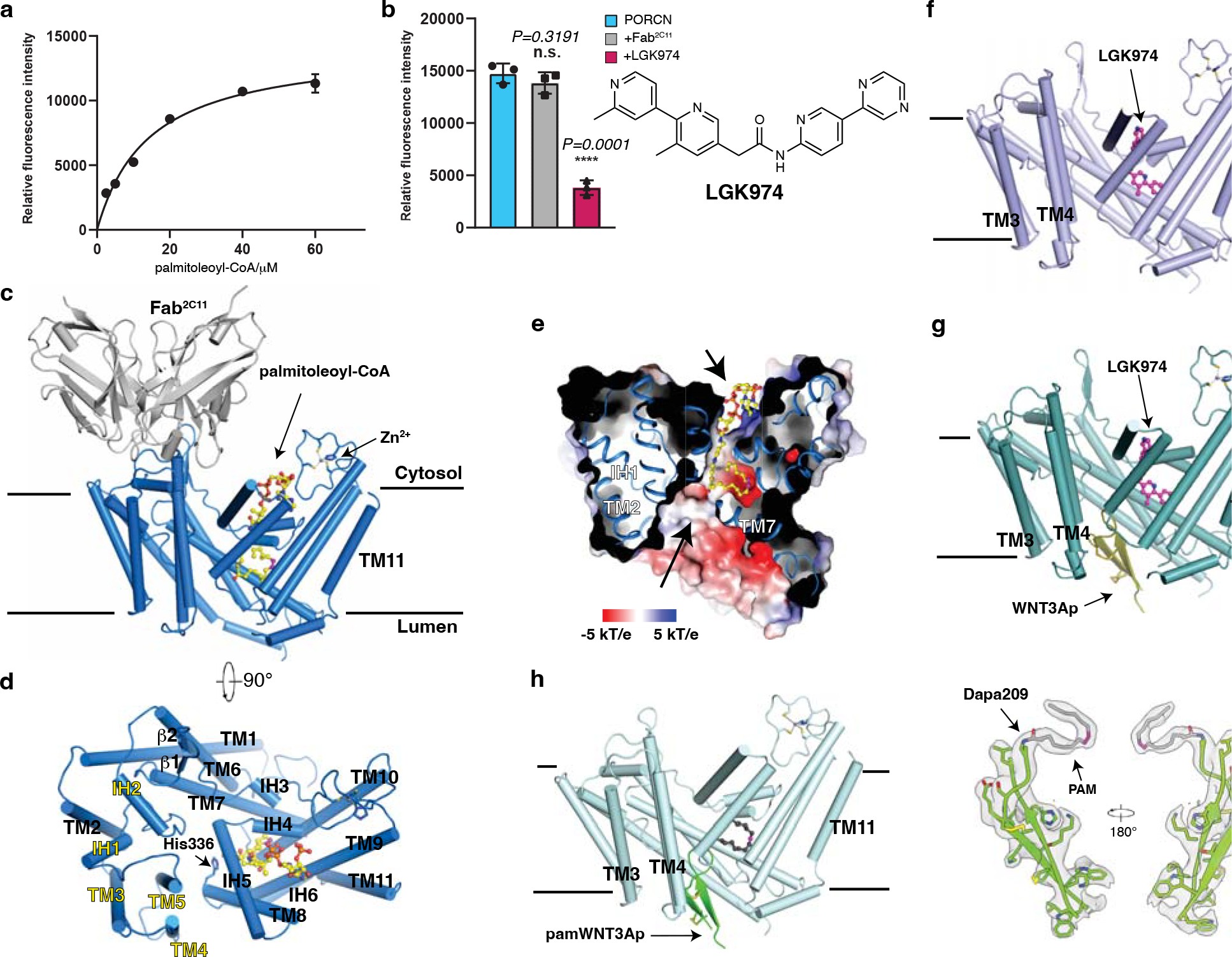
a, Concentration curve of the WNT3Ap acylation by PORCN with palmitoleoyl-CoA (Km=14.29 μM, fitted and calculated using GraphPad Prism 8). Data are mean ± s.d. (n=3 biologically independent experiments). b, LGK974 but not Fab2C11 inhibits the activity of PORCN in vitro. The chemical structure of LGK974 is shown on the right. Data are mean ± s.d. (n=3 biologically independent experiments). ****P ≤ 0.0001, two-tailed unpaired t-test using GraphPad Prism 8. c and d, Overall structure showing palmitoleoyl-CoA-bound PORCN viewed from the side of the membrane (c) or from the cytosol (d). The structural elements that bind to Fab2C11 are highlighted in yellow. e, Electrostatic surface representation of catalytic cavity viewed from the side of the membrane. The routes of substrates access are indicated by arrows. f, Overall structure showing LGK974-bound PORCN. g, Overall structure showing WNT3Ap-bound PORCN in the presence of LGK974. h, Overall structure showing pamWNT3Ap-bound PORCN. The cryo-EM map of pamWNT3Ap is shown. The palmitoleoyl-CoA is shown as sticks in yellow and LGK974 is shown as sticks in magenta. The cis double bond at C9 position of palmitoleoyl chain is colored in magenta. PAM, palmitoleic acid.
