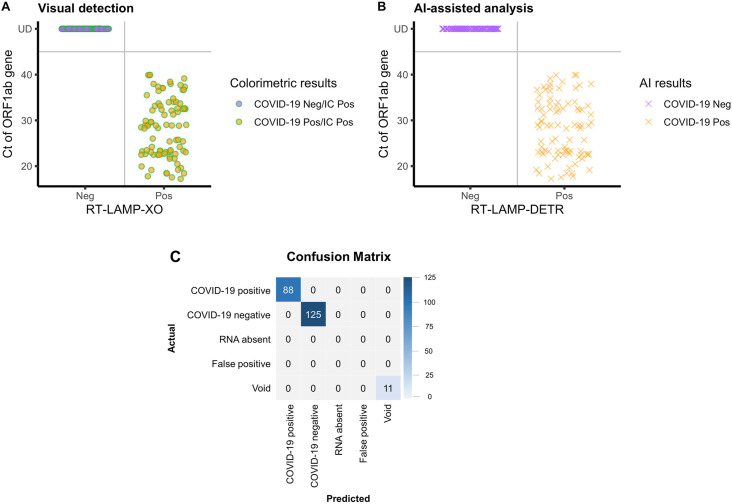
Fig. 5.
Clinical validation of the colorimetric COVID-19 assays by visual detection and RT-LAMP-DETR. The total of 213 total RNA extracted from samples collected by nasopharyngeal swab were subject to COVID-19-RT-LAMP-XO and IC-RT-LAMP-LG assays. The colorimetric results were determined by two modes of analysis: (A) naked-eye observation and (B) automated RT-LAMP-DETR. A Distribution of colorimetric results of the combined COVID-19-RT-LAMP-XO and IC-RT-LAMP-LG assays with respect to the Ct values of RT-qPCR performed on the ORF1ab gene and N gene. All test samples were determined positive by the IC-RT-LAMP-LG assay. B Distribution of automated predictions of the RT-LAMP-DETR model with respect to the Ct values. C Confusion matrix of automated results generated by RT-LAMP-DETR, which simultaneously analyzes the colorimetric outcomes of both COVID-19-RT-LAMP-XO and IC-RT-LAMP-LG assays, and categorizes the test results according to the detected colors of reaction tubes of individual samples. Diagnostic outcomes were compared with RT-qPCR results (Ct value), and the concordance of test results is summarized in Table 1. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)