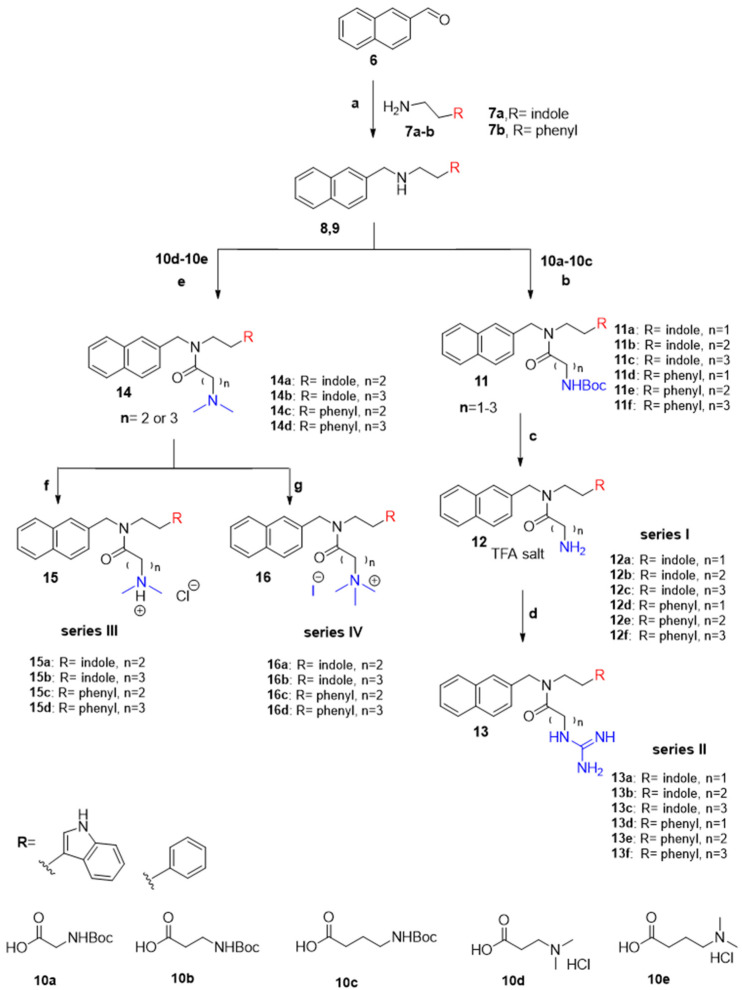
Scheme 1.
General synthetic scheme to synthesize naphthyl-based peptoids. Reaction conditions (a) 7a or 7b (1 equiv.), TMOF, rt, 1.5 h, AcOH, NaCNBH3 (0.3 equiv.) 20 min rt. (b) 10a–10c (1.0–2.0 equiv.), coupling reagent (HATU 1.1 equiv.), (EDC 3.0 equiv.) or (HBTU 2.0 equiv.), DIPEA (3.0 equiv.) or Et3N (3.0 equiv.), DMF, rt, 2–3 h. (c) DCM (1–3 mL), (0.1–0.3 mL) of thioanisole (TA) and 1,2-ethanedithiol (EDT), TFA (1–3 mL). rt, 1–3 h. (d) pyrazole-1H-carboxamidine. HCl (1.0 equiv.), DIPEA (3.0 equiv.) and DMF stir at rt overnight. (e) 10d or 10e (1.1 equiv.), HATU (1.1 equiv.) and DMF rt, overnight. (f) 1 N HCl, rt, 30 min. (g) CH3I (2.0 equiv.), CH3CN, rt, overnight.