Table 1.
Antioxidants frequently used in laboratory experiments.
| Antioxidant | Structure | Protective Effect | Note |
|---|---|---|---|
| Ascorbic acid |
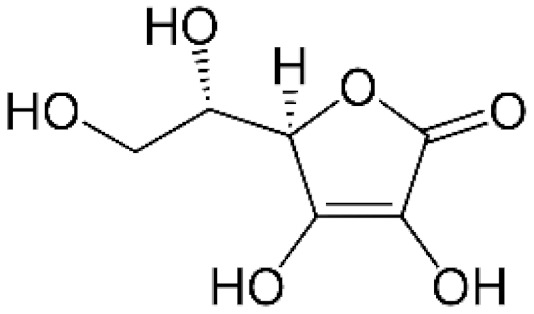
|
Directly interacts with O2•− and H2O2. | In the presence of iron, it becomes powerful source of ROS. |
| Ebselen |
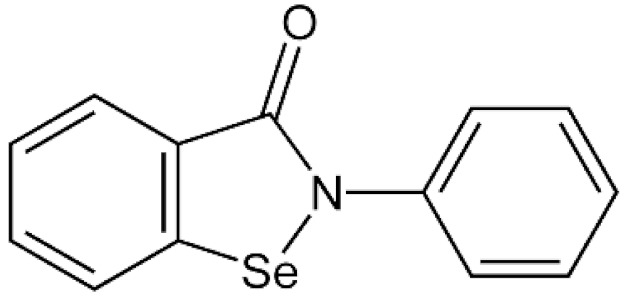
|
Directly interacts with H2O2 at low concentrations. | Ebselen becomes powerful source of ROS at high concentrations. |
| Deferoxamine |
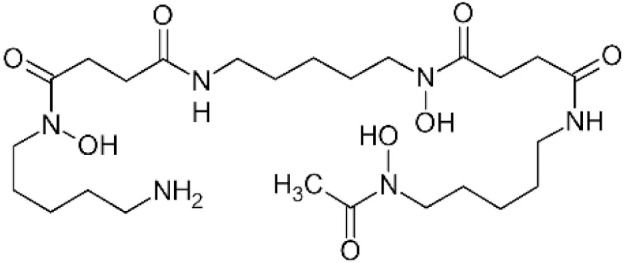
|
As an iron chelator, inhibits Fenton reaction. | Deferoxamine prevents formation of •OH indirectly. |
| 1,4-Dithiothreitol |
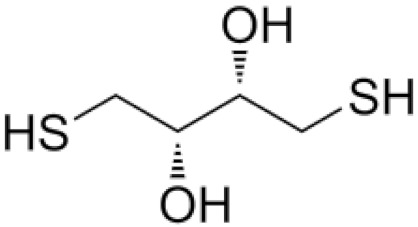
|
Directly interacts with •OH and reduces disulphide bonds in protein samples. | At neutral and alkaline pH, it autoxidises rapidly. It is used in cell-free extracts. |
| Glutathione |
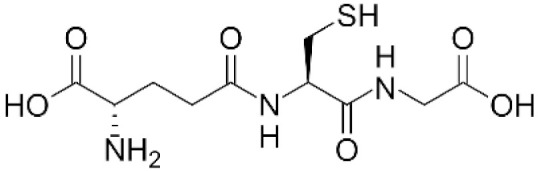
|
Directly interacts with •OH but not with O2•− and H2O2. | In reactions catalysed by GPx, effectively reduces H2O2 and ROOH. |
| Mercaptoethanol |

|
Directly interacts with •OH and reduces disulphide bonds in protein samples. | Due to its relatively high cytotoxicity, it is used in cell-free extracts. |
| N-acetylcysteine |
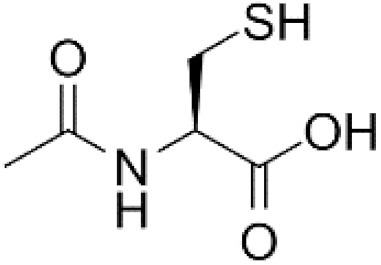
|
Directly interacts with •OH and HOCl but not with O2•− and H2O2. | Although its application scale is limited, it is overused. |
| Thiourea |
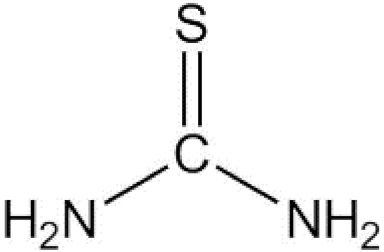
|
Directly interacts with O2•−, H2O2, and •OH. | Although its application scale is very wide, it is used rarely. |
| Trolox |
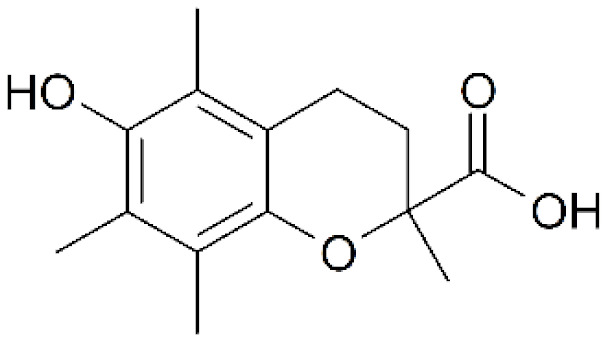
|
It is used to “repair” a variety of biomolecules damaged by oxidative stress. | A water-soluble analogue of vitamin E. |
