Figure 1.
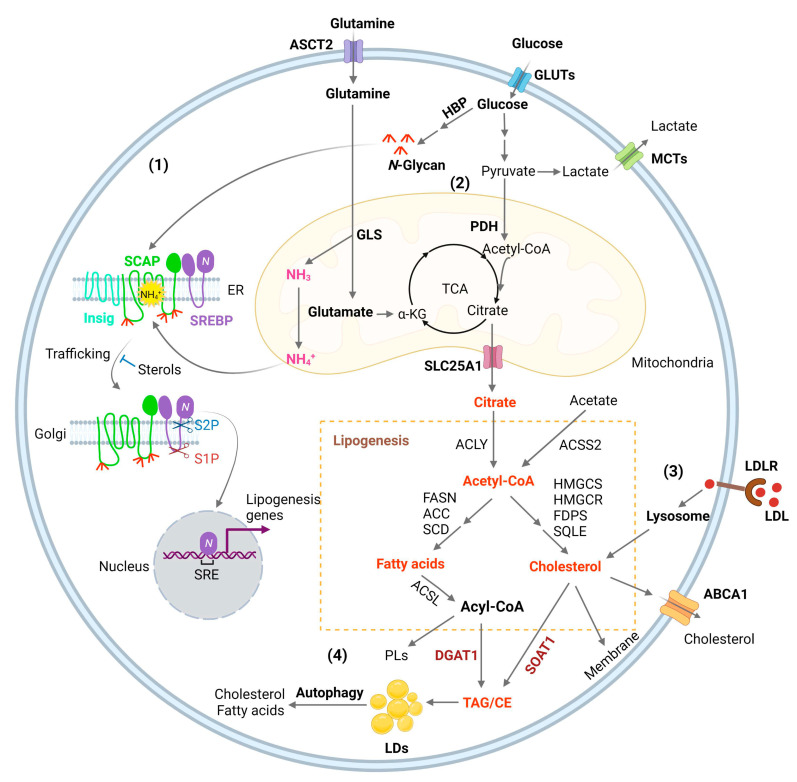
Lipid metabolism reprogramming in cancer cells. (1) Combined elevation of glucose and glutamine consumption promotes lipogenesis by activating the SREBP/SCAP pathway. Glucose produces N-glycans through HBP (Hexosamine Biosynthesis Pathway), which stabilizes SCAP (SREBP-cleavage activating protein) through N-linked glycosylation of its luminal loops (1 and 7). Glutamine enters the mitochondria and ammonia (NH3) is released by GLS (glutaminase). NH3 is protonated and converted to NH4+, which directly binds to the aspartate D428 and Serine S326/330 in the core of the SCAP transmembrane domains, forming stable hydrogen bonds. This binding triggers a dramatic conformation change in SCAP, leading to its dissociation from Insig (insulin-induced gene), an endoplasmic reticulum (ER)-resident protein. Subsequently, SCAP escorts SREBP (sterol regulatory element-binding protein) to the Golgi, where it is cleaved by two enzymes S1P (site 1 protease) and S2P (site 2 protease) to release its active N-terminal fragment. Finally, the N-terminal domain goes into the nucleus, binds to the SRE (sterol regulatory element) motif located in the promoters of gene involved in lipogenesis to activate their transcription and promote de novo lipid synthesis and tumor growth. (2) Glucose is the main source for lipid synthesis. Glucose via glycolysis breaks down into pyruvate, which enters the mitochondria and is converted to acetyl-CoA by PDH (pyruvate dehydrogenase), followed by condensation with OAA (oxaloacetate) to form citrate to enter the TCA cycle (tricarboxylic acid cycle). Citrate is released to the cytosol via its mitochondria transporter, SLC25A1. Citrate is then cleaved to acetyl-CoA by ACLY (ATP citrate lyase), which serves as a precursor for fatty acid and cholesterol biosynthesis catalyzed by a series of enzymes that are the main transcriptional targets of SREBPs, as shown in the Figure. In addition, cytosol acetate can be converted to acetyl-CoA for lipid synthesis by ACCS2 (acetyl-CoA synthetase 2). Besides that, acetyl-CoA is also the substrate for the acetylation of histones, which is involved in epigenetic regulation. Moreover, glutamine through glutaminolysis contributes as an anaplerotic substrate to replenish tricarboxylic acid (TCA) cycle intermediates. Glutamate, the product of the first step of glutaminolysis, is converted to α-KG (α-ketoglutaric acid) and then enters into the TCA cycle. (3) SREBPs upregulates the expression of LDLR (low-density lipoprotein receptor), which binds to LDL and transports it into cells through the endocytosis process. LDL is then hydrolyzed in the lysosomes and cholesterol is released, promoting tumor growth. (4) Excess fatty acids and cholesterol are converted to TAG (triacylglycerol) and CE (cholesteryl ester) by DGAT1 (diacylglycerol O-acyltransferase 1) and sterol O acyl-transferase 1 (SOAT1) to form LDs (lipid droplets) and prevent toxicity from high lipid levels. Under conditions of nutrient deficiency, LDs are hydrolyzed by autophagy to release free fatty acids and cholesterol for tumor survival.