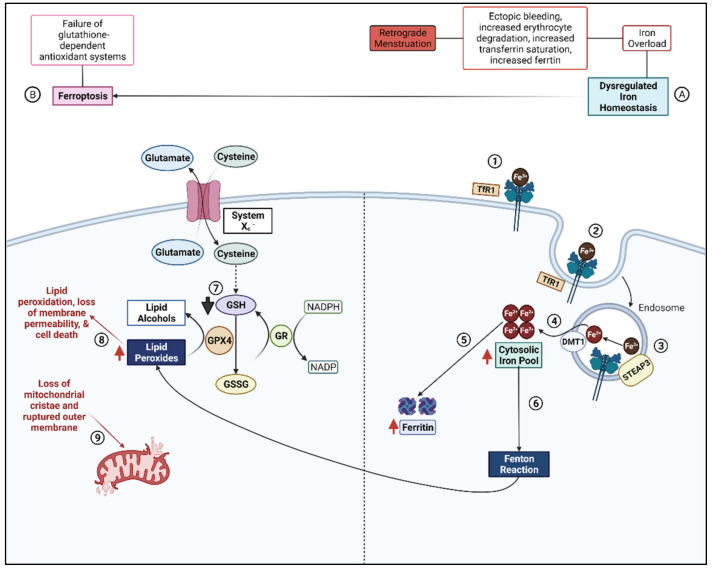
Figure 1.
Dysregulated Iron Homeostasis Leads to Oxidative Stress and Ferroptosis in Endometriosis. (A) Retrograde menstruation, a proposed mechanism for disruption of iron homeostasis in endometriosis, induces ectopic bleeding in the peritoneal cavity (PC). This causes erythrocyte degradation which releases free iron. High levels of iron promote increased transferrin saturation. (1) Transferrin binds to iron and delivers it to tissues where it binds to the transferrin receptor (TfR1). (2) The TfR1-Fe3+ complex enters the cell in an (3) endosome where acidification enables the separation of ferric iron (Fe3+) from the receptor. Six transmembrane epithelial antigens of the prostate 3 (STEAP3) catalyzes the conversion of ferric iron to ferrous iron (Fe2+). (4) Ferrous iron leaves the endosome and accumulates in the cytosolic iron pool. (5) Increased levels of iron in the cytosolic pool leads to increased levels of the intracellular iron storage protein, ferritin. (6) A portion of excess iron will undergo the Fenton reaction to generate free radicals which can damage lipids, proteins, and DNA. (B) This schematic represents a simplified diagram of ferroptosis, an-iron dependent mechanism of cell death. Ferroptosis occurs when glutathione-dependent antioxidant systems fail due to the accumulation of excessive reactive oxygen species (ROS). (7) There is decreased availability of reduced glutathione (GSH) due to excessive intracellular ROS. GSH is required for the conversion of lipid peroxides to non-toxic lipid alcohols by the enzyme glutathione peroxidase (GPX4). (8) Lipid peroxides accumulate and reduce membrane permeability and (9) promote the loss of mitochondrial cristae which contributes to mitochondrial outer membrane rupture. The intracellular accumulation of lipid peroxides and decreased total antioxidant capacity (TAC) eventually leads to cell death. Adapted from “Role of Lipin-1 in Modified-LDL Induced Pro-inflammatory Response”, by Biorender.com (2022). Retrieved from https://app.biorender.com/biorender-templates (accessed on 18 July 2022).