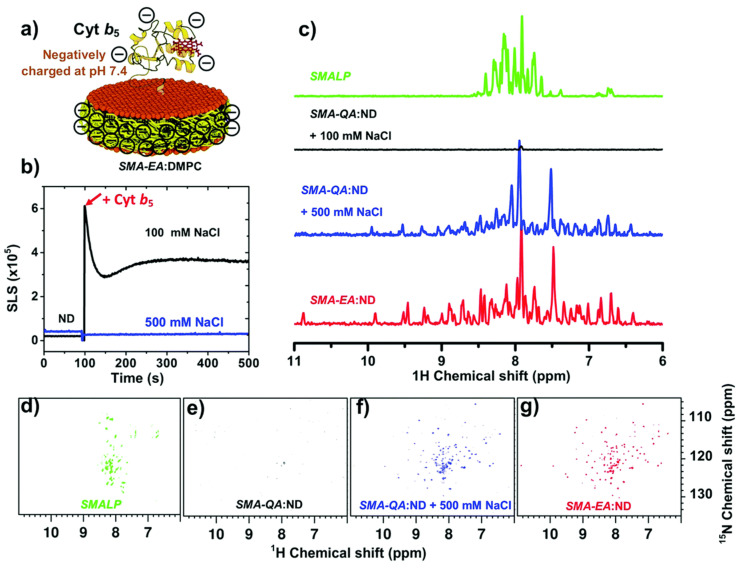
Figure 12.
Reconstitution and structural characterization of cytochrome-b5 in various SMA-based DMPC-nanodiscs: (a) schematic representation of a negatively charged ~16 kDa rabbit cytochrome-b5 reconstituted in negatively charged SMA-EA-based DMPC-nanodiscs. (b) Static light scattering (SLS) profiles of cationic SMA-QA-based DMPC-nanodiscs containing cytochrome-b5 at low (100 mM) and high (500 mM) NaCl concentrations. (c) Projections of 2D 1H/15N TROSY-HSQC NMR spectra of a uniformly-15N-labelled cytochrome-b5 reconstituted in SMALP (d), SMA-QA with 100 mM NaCl (e), SMA-QA with 500 mM NaCl (f), and SMA-EA (g) DMPC-nanodiscs. The presence of aggregates in the sample containing 100 mM NaCl, indicated by the SLS profile in (b), explains the reason for the absence of resonances in the 2D NMR spectrum (e). On the other hand, the appearance of NMR resonance in (f) (and the SLS profile) due to the use of a high concentration of NaCl confirms the formation of non-specific charge-charge coulombic interactions between the positively-charged SMA-QA polymer belt and the negatively-charged cytochrome-b5. Although the use of high salt concentration enables NMR data acquisition, it is not physiologically relevant. It can damage proteins in NMR samples by causing serious radio-frequency-induced heating in the sample to. This Figure and caption are adopted from reference [199].