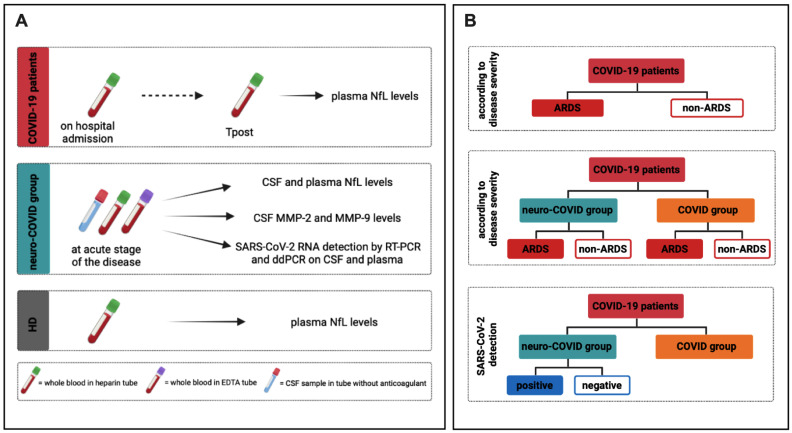
Figure 1.
Study design. (A) For COVID-19 patients, whole blood samples were collected in heparin tubes during routine clinical testing at two timepoints: on hospital admission (baseline) and after three months from discharge (Tpost). For the neuro-COVID group, CSF samples and heparin and EDTA whole blood samples were collected. RT-PCR and ddPCR were used for the detection and quantification of SARS-CoV-2 RNA in collected CSF and whole blood samples. For healthy donors (HD) heparin whole blood samples were collected. (B) According to clinical outcome, COVID-19 patients were stratified into ARDS and non-ARDS groups and the differences in plasma NfL levels were evaluated. According to disease severity, both neuro-COVID group and COVID groups were stratified into ARDS and non-ARDS groups and the differences in CSF NfL levels, plasma NfL levels, and CSF MMP-2 and MMP-9 levels were assessed. According to the detection of SARS-CoV-2 RNA in CSF and plasma samples, neuro-COVID group was stratified into positive and negative groups and the differences in CSF NfL levels as well as MMP-2 and MMP-9 levels were evaluated. Neuro-COVID group: COVID-19 patients with severe neurological symptoms; COVID group: COVID-19 patients without severe neurological symptoms; NfL: neurofilament light chain; MMP-2: matrix metalloprotease-2; MMP-9: matrix metalloprotease-9; SARS-CoV-2: severe acute syndrome coronavirus 2; RT-PCR: reverse transcription-polymerase chain reaction; ddPCR: droplet digital polymerase chain reaction; CSF: cerebrospinal fluid; HD: healthy donors; EDTA: ethylenediaminetetraacetic acid; ARDS: acute respiratory distress syndrome.