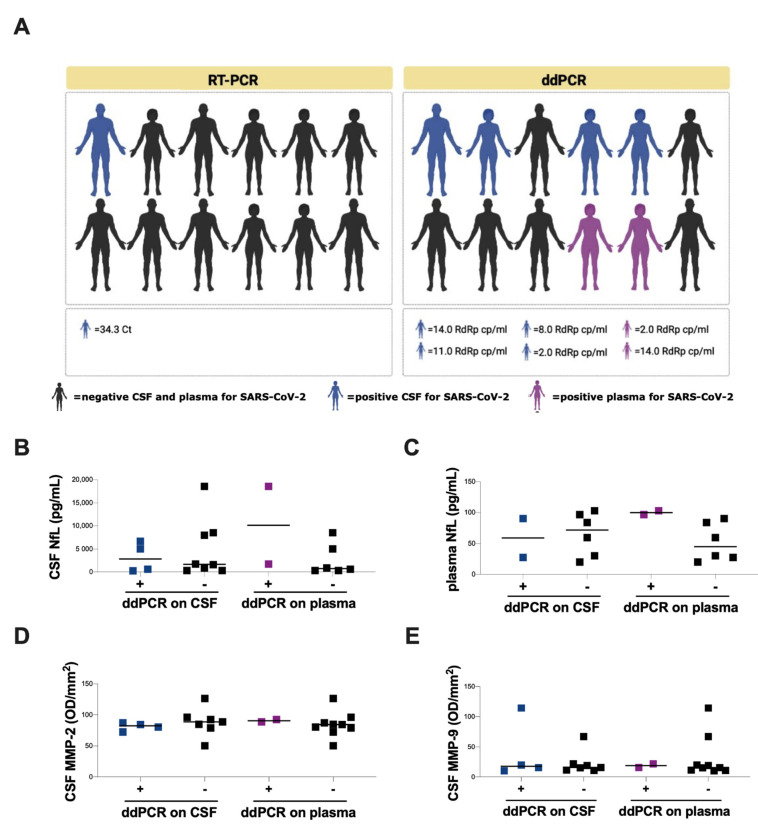
Figure 4.
Detection of SARS-CoV-2 RNA on CSF and plasma samples and evaluation of NfL and MMPS levels in neuro-COVID group. (A) Among COVID-19 patients with neurological symptoms (neuro-COVID group), the detection of SARS-CoV-2 RNA on CSF and plasma samples was performed using RT-PCR and ddPCR. (B) Evaluation of CSF NfL levels in COVID-19 patients with severe neurological symptoms (neuro-COVID group) stratified according to ddPCR positivity on CSF and plasma. (C) Evaluation of plasma NfL levels in COVID-19 patients with severe neurological symptoms (neuro-COVID group) stratified according to ddPCR positivity on CSF and plasma. (D) Evaluation of CSF MMP-2 levels in COVID-19 patients with severe neurological symptoms (neuro-COVID group) stratified according to ddPCR positivity on CSF and plasma. (E) Evaluation of CSF MMP-9 levels in COVID-19 patients with severe neurological symptoms (neuro-COVID group) stratified according to ddPCR positivity on CSF and plasma. Horizontal lines represent medians. SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; RT-PCR: reverse transcription-polymerase chain reaction; ddPCR: droplet digital polymerase chain reaction; CSF: cerebrospinal fluid; NfL: neurofilament light chain; MMP-2: matrix metalloprotease-9; MMP-9: matrix metalloprotease-9.