Abstract
Simple Summary
Tumor necrosis factor (TNF) receptor associated factor-2 (TRAF2) is an intracellular adapter protein with E3 ligase activity, which interacts with a plethora of other signaling proteins, including plasma membrane receptors, kinases, phosphatases, other E3 ligases, and deubiquitinases. TRAF2 is involved in various cancer-relevant cellular processes, such as the activation of transcription factors of the NFκB family, stimulation of mitogen-activated protein (MAP) kinase cascades, endoplasmic reticulum (ER) stress signaling, autophagy, and the control of cell death programs. In a context-dependent manner, TRAF2 promotes tumor development but it can also act as a tumor suppressor. Based on a general description, how TRAF2 in concert with TRAF2-interacting proteins and other TRAF proteins act at the molecular level is discussed for its importance for tumor development and its potential usefulness as a therapeutic target in cancer therapy.
Abstract
Tumor necrosis factor (TNF) receptor associated factor-2 (TRAF2) has been originally identified as a protein interacting with TNF receptor 2 (TNFR2) but also binds to several other receptors of the TNF receptor superfamily (TNFRSF). TRAF2, often in concert with other members of the TRAF protein family, is involved in the activation of the classical NFκB pathway and the stimulation of various mitogen-activated protein (MAP) kinase cascades by TNFRSF receptors (TNFRs), but is also required to inhibit the alternative NFκB pathway. TRAF2 has also been implicated in endoplasmic reticulum (ER) stress signaling, the regulation of autophagy, and the control of cell death programs. TRAF2 fulfills its functions by acting as a scaffold, bringing together the E3 ligase cellular inhibitor of apoptosis-1 (cIAP1) and cIAP2 with their substrates and various regulatory proteins, e.g., deubiquitinases. Furthermore, TRAF2 can act as an E3 ligase by help of its N-terminal really interesting new gene (RING) domain. The finding that TRAF2 (but also several other members of the TRAF family) interacts with the latent membrane protein 1 (LMP1) oncogene of the Epstein–Barr virus (EBV) indicated early on that TRAF2 could play a role in the oncogenesis of B-cell malignancies and EBV-associated non-keratinizing nasopharyngeal carcinoma (NPC). TRAF2 can also act as an oncogene in solid tumors, e.g., in colon cancer by promoting Wnt/β-catenin signaling. Moreover, tumor cell-expressed TRAF2 has been identified as a major factor-limiting cancer cell killing by cytotoxic T-cells after immune checkpoint blockade. However, TRAF2 can also be context-dependent as a tumor suppressor, presumably by virtue of its inhibitory effect on the alternative NFκB pathway. For example, inactivating mutations of TRAF2 have been associated with tumor development, e.g., in multiple myeloma and mantle cell lymphoma. In this review, we summarize the various TRAF2-related signaling pathways and their relevance for the oncogenic and tumor suppressive activities of TRAF2. Particularly, we discuss currently emerging concepts to target TRAF2 for therapeutic purposes.
Keywords: apoptosis, autophagy, B-cell lymphoma, cellular inhibitor of apoptosis 1/2 (cIAP1/2), necroptosis, nuclear factor ‘kappa-light-chain-enhancer’ of activated B-cells (NFκB), tumor necrosis factor (TNF), TNF receptor associated factor 2 (TRAF2)
1. Introduction
In pioneering work in the mid-1990s, the group of D. Goeddel identified four proteins recruiting to tumor necrosis factor (TNF) receptor 2 (TNFR2). Two of these proteins indicated homology to the just previously identified baculovirus-encoded inhibitor of apoptosis proteins and were accordingly named cellular inhibitor of apoptosis 1 (cIAP1) and -2 (cIAP2) [1]. The two other proteins demonstrated no homologies to proteins known at that time, but shared a conserved C-terminal stretch of app. 200 aa. The two proteins have been named TNF receptor-associated factor-1 (TRAF1) and -2 (TRAF2) and the C-terminal homology domain accordingly as TRAF domain [2]. A C-terminal TRAF domain has also been discovered in four other human proteins, named TRAF3 to TRAF6, and has been subdivided in the compact coiled-coil TRAF-N domain mediating trimerization, and the more loosely packed TRAF-C domain, which in the case of TRAF2 mediates binding to a short aa motif in the cytoplasmic domain of TNFR2 (Figure 1). With exception of TRAF1, the TRAF proteins also share a common N-terminal domain architecture composed of an interesting new gene (RING) domain followed by 5-7 zinc fingers. While activated TNFR2 directly binds TRAF2 and TRAF1, cIAP1 and cIAP2 are indirectly recruited to TNFR2 by help of TRAF2. In fact, eventually TRAF2 seems to fulfill many of its functions in concert with these proteins.
Figure 1.
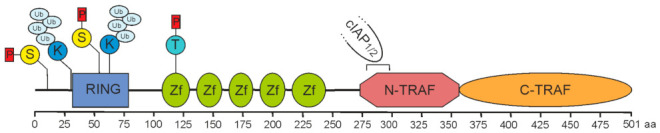
Domain architecture of TRAF2. A sequence of 501 amino acids prescribes the molecular structure of full-length TRAF2. It is essentially composed of a RING domain (aa 32–79), five zinc fingers (Zf) and a TRAF domain comprising a coiled-coil N-TRAF domain with a cIAP1/2 binding site (aa 283–294) [3,4] and a C-TRAF domain. Phosphorylation and ubiquitination sites of known relevance for TRAF2 function are indicated and comprise serine S11 and S55 [5,6,7], lysine K31 and K63 [8,9], and threonine T117 [10].
Early after its identification as part of the TNFR2 signaling complex, it has been recognized that TRAF2 is also recruited to the majority of other receptors of the TNF receptor superfamily (TNFRSF) including nearly all TNFRSF receptors (TNFRs) with a TRAF binding motif (direct TRAF2 binding, see Table 1) and all TNFRs with a death domain (DD) (indirect TRAF2 binding). Later, it also became evident that TRAF2 acts beyond the TNFRSF in the signal transduction by other immune regulatory receptors, including members of the toll-like receptor (TLR) family, the type I interferon receptor and the retinoic acid-inducible gene I (RIG I)-like receptor (RLR) family of intracellular pattern recognition receptors recognizing viral RNA (Table 1). Finally, yet importantly, TRAF2 has been implicated in autophagy and endoplasmic reticulum (ER) stress signaling. TRAF2 fulfills its functions primarily by acting as a scaffold, which in a signal-inducible or constitutive manner brings together E3 ligases, their substrates, and also a variety of regulatory factors, including deubiquitinating enzymes (Table 1).
Table 1.
TRAF2 interacting proteins.
| Protein | Type of Protein | Experimental Evidence |
Target Domain in TRAF | Reference |
|---|---|---|---|---|
| TNFR2 | TNFRSF | THS, GST, endo Co-IP | CTD | [2] |
| LTβR | TNFRSF | endo Co-IP | [11] | |
| OX40 | TNFRSF | THS, Co-IP | [12,13] | |
| CD40 | TNFRSF | THS, GST, endo Co-IP | [14] | |
| CD27 | TNFRSF | THS, Co-IP | [15,16,17] | |
| CD30 | TNFRSF | THS, GST | [18,19] | |
| 4-1BB | TNFRSF | THS, GST, endo Co-IP | [12,20,21] | |
| RANK | TNFRSF | GST, Co-IP | [22,23,24] | |
| Fn14 | TNFRSF | GST | [25] | |
| TACI | TNFRSF | THS, Co-IP | [26] | |
| HVEM | TNFRSF | GST | [27] | |
| NGFR | TNFRSF | Co-IP | [28] | |
| BCMA | TNFRSF | Co-IP | [29] | |
| GITR | TNFRSF | THS, endo Co-IP | [30,31] | |
| TROY | TNFRSF | Co-IP | [32] | |
| IL15R | receptor | Co-IP | [33] | |
| IFNαR1 | receptor | GST, Co-IP | [34] | |
| LMP1 | viral oncogen | GST, Co-IP | [35,36] | |
| A20 | DUB, E3 ligase | THS, Co-IP | [37] | |
| AIP1 | Ras-GAP | Co-IP | RING/zinc | [38] |
| AIMP2 | adaptor | THS, GST, endo Co-IP | [39] | |
| APPL1 | adaptor | GST, endo Co-IP | [40] | |
| AWP1 | adaptor | THS, Co-IP | TD | [41] |
| Bcl10 | adaptor | THS, Co-IP | [42] | |
| Beclin | autophagy | GST, endo Co-IP | RING | [43] |
| Caspase-2 | caspase | Endo Co-IP | [44] | |
| Caspase-12 | caspase | Co-IP | NTD | [45] |
| β-catenin | proto-oncogene | Co-IP, MST | TD | [46] |
| caveolin-1 | plasma membrane protein | endo Co-IP | [47] | |
| CHIP | E3 ligase | endo Co-IP | [48] | |
| cIAP1 | E3 ligase | THS, Co-IP | NTD | [1] |
| cIAP2 | E3 ligase | THS, Co-IP | NTD | [1] |
| CYLD | DUB | THS, Co-IP | TD | [49] |
| DUSP14 | phosphatase | Co-IP | [50] | |
| DYRK1A | kinase | endo Co-IP | TD | [51] |
| EGFR | kinase | endo Co-IP | [52] | |
| EI24 | E3 ligase | Co-IP | [53] | |
| eIF4GI | scaffold | THS, GST, Co-IP | TD | [54] |
| Eva1 | adhesion protein | endo Co-IP | [55] | |
| FAK | kinase | endo Co-IP | [56] | |
| Filamin | actin binder | Co-IP | RZ | [57] |
| GCKR | kinase | endo Co-IP | TD | [58] |
| Gpx1 | peroxidase | Co-IP | TD | [59] |
| GRA15 | virulence factor | Co-IP | [60] | |
| GSTP1-1 | gluthation transferase | Co-IP | [61] | |
| HGK | Kinase | Co-IP | [62] | |
| Hoxa1 | transcription factor | THS, Co-IP | [63] | |
| HSP70 | Chaperon | Co-IP | TD | [64] |
| IKK1 | kinase | GST, endo Co-IP | RING | [65] |
| IKK2 | kinase | GST, endo Co-IP | RING | [65] |
| IKKe | kinase | Co-IP | [66] | |
| IRE1 | kinase, nuclease | endo Co-IP | [67] | |
| I-TRAF | adaptor | THS, GST, Co-IP | TD | [68] |
| JIK | kinase | Co-IP | [45] | |
| KRC | DNA binding | endo Co-IP | TD | [69] |
| LGP2 | RLR | Co-IP | CTD | [70] |
| LILRB3 | receptor | endo Co-IP | [71] | |
| LRPPRC | RNA regulation | Co-IP | [72] | |
| MAVS | adaptor | Co-IP | CTD | [73,74] |
| MEKK1 | kinase | Co-IP | [75] | |
| MIZ | transcription factor | GST, endo Co-IP | RING | [76] |
| MLK3 | kinase | Co-IP | NTD | [77,78] |
| MST1 | kinase | endo Co-IP | Zn fingers | [79] |
| TRIM37 | E3 ligase | Co-IP | TD | [80] |
| Nef | virulence factor | GST | [81,82] | |
| HCV core | virulence factor | GST | [81] | |
| NIP45 | transcription factor associated | endo Co-IP | [83] | |
| NSP1 | virulence factor | Co-IP | [84] | |
| Nur77 | nuclear receptor | Co-IP | RING, NTD | [85] |
| parkin | E3 ligase | endo Co-IP | [86] | |
| proPTPRN2 | phosphatase | Co-IP | RING | [87] |
| RET/PTC3 | oncogenic RTK fusion protein | Co-IP | [88] | |
| RIPK1 | kinase | Co-IP | NTD, CTD | [89] |
| RIP2 | kinase | Co-IP | [90] | |
| RNAseT2 | ribonuclease | Co-IP | [91] | |
| RSK2 | kinase | Co-IP | [92] | |
| SHP-1 | phosphatase | Co-IP | [93] | |
| SGEF | GEF | Co-IP | TD | [94] |
| Sharpin | scaffold | Co-IP | [95] | |
| SIAH-2 | E3 ligase | GST | [96] | |
| SMAD4 | signaling protein | THS, endo Co-IP | [97] | |
| SMURF-2 | E3 ligase | THS, Co-IP | [98] | |
| SMYD2 | methyltransferase | Co-IP, SPR | [99] | |
| SphK1 | kinase | GST, Co-IP | [100] | |
| T2BP / TIFA | adaptor | THS, Co-IP | TD | [101] |
| TCPTP | phosphatase | endo Co-IP | [102] | |
| TPL2/COT1 | kinase | Co-IP | [103] | |
| TAK1 | kinase | Co-IP | [104] | |
| TBK1 | kinase | endo Co-IP | NTD | [74] |
| TNIK | kinase | Co-IP | TD | [105] |
| TRADD | adaptor | THS, Co-IP | CTD | [89,106] |
| TRAF1 | adaptor | THS, Co-IP | NTD, CTD | [2,89] |
| TRAF2 | E3 ligase, adaptor | THS, Co-IP | CTD | [2,89] |
| TRAF3 | E3 ligase, adaptor | Co-IP | [107] | |
| TRAF4 | E3 ligase, adaptor | endo Co-IP | [108] | |
| TRIF | adaptor | THS, Co-IP | [109] | |
| UBC13 | E2 | endo Co-IP | RING | [76] |
| USP4 | DUB | Co-IP | [110] | |
| USP7 | DUB | GST | [80] | |
| USP17 | DUB | Co-IP | [107] | |
| UXT-V1 | transcriptional cofactor | endo Co-IP | [111] | |
| VP4 | rotavirus capsid protein | THS, Co-IP | [112] | |
| WDR62 | scaffold | Co-IP | [113] |
Abbreviations: Co-IP, co-immunoprecipitation of transiently expressed proteins; CTD, C-TRAF domain; endo Co-IP, co-immunoprecipitation of endogenous proteins; DUB, de-ubiquitinase; GST, glutathione-S transferase pull-down assay; NTD, N-TRAF domain; RING domain; TD, TRAF domain; THS, two-hybrid system; zinc, zinc finger domain.
The most important TRAF2-interacting E3 ligases are cIAP1 and cIAP2. Prominent substrates of the TRAF2-cIAP1/2 complex are kinases, such as transforming growth factor-β (TGF-β)-activated kinase 1 (TAK1), NFκB-inducing kinase (NIK), apoptosis signal-regulating kinase 1 (ASK1), and receptor-interacting kinase 1(RIPK1), which regulate NFκB signaling and induction of programmed cell death. Intriguingly, in some special cases TRAF2 may also act itself as an E3 ligase by help of its RING domain, such as in context of TRAIL death receptor signaling where TRAF2 K48-ubiquitinates caspase-8 [114,115]. Like most other TRAF family members, TRAF2 is involved in the engagement of signaling pathways resulting in the activation of transcription factors, such as the two NFκB pathways, various mitogen-activated protein (MAP) kinase cascades, and the MAVS/TBK1/IRF3 pathway. However, TRAF2 can also affect cellular functions independent from transcription-stimulating pathways by triggering phosphorylation and/or ubiquitination of proteins, thereby regulating their activity, stability, function, or localization. Examples therefore are K48-ubiquitiantion and proteasomal degradation of caspase-8, cRel, interferon regulatory factor 5 (IRF5), and unc-51-like autophagy activating kinase 1 (ULK1) triggered alone by TRAF2 (caspase-8) or by TRAF2 in concert with TRAF3 and cIAP1 and cIAP2 (cRel, IRF5, ULK1) [114,115,116,117]. Further examples of “transcription”-independent TRAF2 activities are the engagement of the Src homology 3 domain-containing guanine nucleotide exchange factor (SGEF), leading to glioblastoma cell migration in response to Fn14 activation [94], and K63-ubiquitination of dual-specificity tyrosine phosphorylation-regulated kinase 1A (DYRK1A), promoting its translocation to vesicles to attenuate epidermal growth factor receptor (EGFR) degradation [51] and its role in mitophagy [85,118,119].
2. Role of TRAF2 in Immune Signaling Pathways
2.1. TRAF2 and Activation of the Classical NFκB Pathway
Nuclear factor kappa-light-chain-enhancer of activated B-cells (NFκB) are homo- and heterodimeric transcription factors formed of the five NFκB proteins p65/RelA, RelB, cRel, p50, and p52, of which the latter two are initially expressed in the form of precursor molecules (p100 and p105). NFκB dimers are held in check by cytoplasmic retention [120,121] resulting from the fact that the nuclear localization sequence (NLS) of NFκBs is blocked in non-stimulated cells by either of two related mechanisms. First, by forming a ternary complex with ankyrin-repeat containing inhibitor of κB proteins (IκBs), e.g., IκBα, or second, by incomplete maturation of the precursor proteins p100 and p105 containing a C-terminal autoinhibitory ankyrin-repeat domain. There are two distinct signaling mechanisms that relieve the NLS of NFκBs from the inhibitory interaction with ankyrin repeats: firstly, the IκB kinase (IKK) complex-induced degradation of IκB proteins and the IKK-induced processing of p105 (classcial or canonical NFκB pathway) and secondly, the NIK-induced processing of p100 to p52 (alternative or non-canonical NFκB pathway) (Figure 2).
Figure 2.
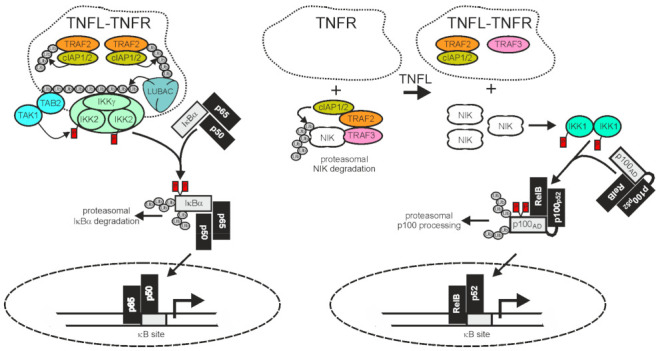
TRAF2 and the cIAPs in receptor-induced activation of the classical and alternative NFκB pathway. The activities of TRAF2 and the cIAPs have opposing qualities in the classical (left panel) and alternative (right panel) NFκB signaling pathway. In the classical NFκB pathway TRAF2 and the cIAPs enable signaling, while in the alternative NFκB pathway they act as inhibitors. Importantly, TNFR-induced recruitment of TRAF2 and the cIAPs, which triggers the classical NFκB pathway, is linked with an “inhibitory” relocation of these molecules away from their cytosolic substrate NIK in the alternative NFκB pathway. Therefore, TNFRs eventually stimulate both NFκB signaling pathways despite the opposing quality they have in these pathways. For more details, refer to main text.
A major function of TRAF2 is to transduce activating signals from cell surface receptors, particularly TNFRs, to the IKK complex in the classical NFκB pathway. The latter phosphorylates IκBα and related IκBs to trigger their proteasomal degradation, the key event in activation of the classical NFκB pathway (Figure 2). To fulfill its tasks in classical NFκB signaling, TRAF2 directly or indirectly recruits to the liganded receptor molecules along with the TRAF2-interacting E3 ligases cIAP1 and cIAP2. This results in the activation of the latter. The cIAPs in turn K63 ubiquitinate TRAF2 and other proteins present in the receptor signaling complexes, and thereby create docking sites facilitating the recruitment of the linear ubiquitin assembly complex (LUBAC). The latter catalyzes the formation of linear M1-linked ubiquitin chains, creating binding sites for the NFκB essential modulator (NEMO), a subunit of the IKK complex, and the TAK1-binding-protein-2 (TAB2) subunit of the IKK-engaging TAB2-TAK1 complex. Worth mentioning, TRAF2 also triggers the recruitment of regulatory proteins, such as deubiquitinases, that terminate/resolve the ubiquitination events leading to IKK activation. For example, the cylindromatosis tumor suppressor (Cyld) directly interacts with TRAF2 and removes K63-linked polyubiquitin chains from TRAF2, resulting in reduced NFκB signaling and enhanced apoptosis but also in the maintenance of hematopoietic stem cell dormancy by inhibition of p38 MAP kinase signaling [49,122,123,124]. Likewise, A20 [also named TNFα-induced protein 3 (TNFAIP3)] acts as a K63 deubiquitinase, e.g., for RIPK1, NEMO/IKKγ, or caspase-8, but also, alone or in concert with other E3 ligases, as a K48 E3 ligase [125]. In accordance with the function of its major substrates and in view of the fact that A20 itself is a NFκB target, A20 has been implicated in the downregulation of the classical NFκB pathway and the control of cell death [125].
The generalized mechanisms of receptor-induced TRAF2-mediated activation of the IKK complex described above have been primarily investigated for TNFR1, but there is evidence that similar or related mechanisms also apply for other receptors. For example, cIAPs and/or the LUBAC have also been implicated in NFκB activation by other TRAF2-utilizing receptors, such as CD40, TNFR2, Fn14, and the TRAIL death receptors [126,127,128,129]. Intriguingly, the NFκB-inhibitory effects of dominant-negative TRAF2 mutants on TNFR signaling reported in early years is often more pronounced than the inhibitory effect observed in receptor stimulated TRAF2-deficient cells. A possible explanation for this is that other TRAF proteins, which use overlapping binding sites to TRAF2 in the considered TNFR type, act redundantly with TRAF2 and/or fulfill functions distinct of those of TRAF2. In fact, there is evidence that TRAF2 and TRAF5 act redundantly in TNF-induced classical NFκB signaling and that TRAF2, in cooperation with TRAF1 and TRAF6, redundantly signal CD40-induced NFκB activation [130,131]. Furthermore, it is well-established that TRAF2 cooperates with TRAF3 in the control of alternative NFκB signaling (see 2.2.). In general, however, redundancy and/or cooperativity between TRAF2 and other TRAF proteins have been limitedly investigated so far.
2.2. TRAF2 and Activation of the Alternative NFκB Pathway
TRAF2 and the cIAPs play a central role in the control of the alternative NFκB signaling pathway. In the cytoplasm TRAF2 interacts via TRAF3 with NIK which is constitutively active and enables cIAP1/2-mediated K48-ubiquitination of the latter, resulting in its proteasomal degradation [132,133]. NIK activates IKKα which in turn phosphorylates p100, triggering its proteasomal processing to p52. Therefore, TRAF2, TRAF3, and the cIAPs finally inhibit the alternative NFκB pathway. Thus, in the classical NFκB pathway, TRAF2 and the cIAPs trigger the degradation of pathway inhibitory ankyrin-repeat proteins or ankyrin-repeat domains (IκBs, ankyrin domain of p105), whereas in the alternative NFκB pathway, the same proteins prevent, together with TRAF3, the degradation of a pathway inhibitory ankyrin-repeat domain (Figure 2). The alternative NFκB pathway is typically engaged by members of the TNFRSF, such as Fn14, CD40, TNFR2, and the LTβR. In view of the opposing effects of TRAF2 and the cIAPs on ankyrin-repeat containing NFκB-inhibitory proteins/protein domains in the two NFκB signaling pathways, it first seems counter-intuitive that activation of TNFRs results in the concomitant activation of both pathways. However, this apparent contradiction is resolved when two points are considered: i) that the amount of cell-expressed TRAF2 and cIAP1/2 molecules is limited and ii) that TRAF2 and the cIAPs, along with TRAF3, act constitutively in the cytoplasm of unstimulated cells in context of the alternative NFκB pathway but fulfill their role in the classical NFκB pathway in an inducible manner in plasma membrane-associated receptor signaling complexes. Ligand-induced recruitment of TRAF2 (and/or TRAF3) and the cIAPs to plasma membrane-receptors is accordingly intimately linked to the depletion of these molecules from the cytosol, resulting not only in the formation of classical NFκB-stimulating receptor complexes but also in a reduction of the cytosolic available amount of TRAF2-cIAP1/2 complexes that can be recruited via TRAF3 to NIK to inhibit the alternative NFκB pathway. It is worth mentioning that the sole depletion of TRAF2, TRAF3, and the cIAPs from the cytoplasm is sufficient to engage the alternative NFκB pathway [132,133,134,135] but that this mechanism can be enhanced in its effects by receptor-associated degradation of the TRAFs and the cIAPs. Taken together, despite the opposing quality of the activity of TRAF2 and the cIAPs on the two NFκB signaling pathways, ligand-induced receptor-TRAF2 interaction eventually results in concomitant activation of both pathways.
2.3. TRAF2 in RLR Signaling
In RNA virus-infected cells cytosolic double-stranded (ds) RNA is recognized by RIG1 and/or the RIG1-like receptor (RLR) melanoma differentiation-associated 5 (MDA5) and laboratory of genetics and physiology 2 (LGP2) [136]. Binding of dsRNA by RIG1 and MDA5 enables these proteins to convert from an autoinhibited form to a tetrameric “open form” which, assisted by the E3 ligase RIPLET and K63-polyubiquitination, assembles into filaments [137,138,139] (Figure 3).
Figure 3.
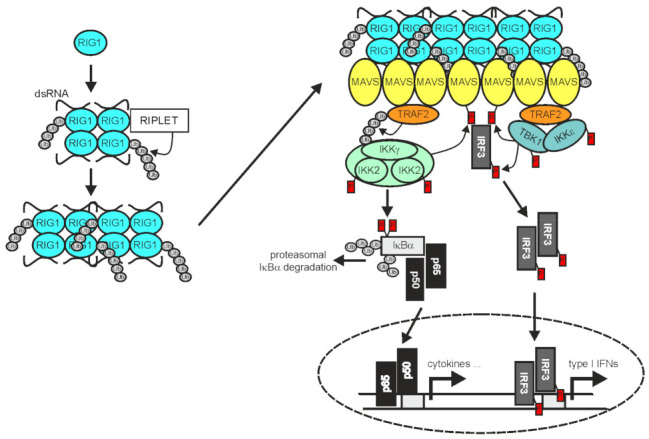
TRAF2 in RIG1 signaling. Binding of dsRNA by RIG1, results in conformational change, K63-ubiquitination, and filament formation. RIG1 filaments in turn instruct filament formation of mitochondria-associated MAVS. The MAVS filaments enable recruitment of TRAF2 and IRF3, but also other TRAF proteins not indicated here. TRAF2 and the other TRAF proteins mediate the recruitment of the IKK complex and TANK-binding kinase 1 (TBK1)/IKKε enabling activation of the classical NFκB pathway and IRF3 by the mechanisms described in detail in the text. Please note, TRAF2 acts independently here from cIAP1 and cIAP2 [74]. For more details refer to main text.
The RLR filaments in turn bind to mitochondria antiviral signaling protein (MAVS; also named VISA, IPS-1, or Cardif) and nucleate the formation MAVS filaments [140]. The latter in turn act as signaling platforms, like aggregated TNFRs, to recruit TRAF2, TRAF3, TRAF5, and TRAF6 along with IRF3 and the TRAF-interacting IKK- and TBK1/IKKε complexes to engage downstream signaling pathways, namely the classical NFκB pathway and the TBK1/IRF3/IFNβ pathway [74,141,142]. TRAF2 and TRAF5 on the one side and TRAF3 and TRAF6 on the other side bind to different binding motifs in MAVS and act redundantly to activate a strong innate immune response [141]. While the RING domains of TRAF2 and the other TRAFs were found to be important to mediate NEMO ubiquitination and IKK activation in context of RLR signaling, they appeared dispensable for activation of the TBK1-IRF3 axis [74]. In contrast to receptors of the TNFRSF, RIG1 and MDA5 not only stimulate the activation of NFκB transcription factors by help of the TRAF proteins but also engage IRF3 and IRF7. The reasons for the different signaling qualities of TRAF2 and the other TRAFs in TNFR versus RLR signaling are still unclear. It is worth mentioning that the RLR LGP2 associates with the C-terminus of TRAF2, TRAF3, TRAF5, and TRAF6 and acts as a pan-inhibitor of stimuli using these TRAF proteins for activation of the classical NFκB pathway [70].
3. TRAF2 in the Control and Integration of Cell Death Programs, ER Stress and Autophagy
3.1. TRAF2 and Programmed Cell Death
Soon after it was discovered that TRAF2 not only interacts with TNFR2 but is also recruited to the death domain (DD)-containing TNFR TNFR1 by virtue of the death domain-containing adapter protein TRADD [106,143], it became evident that TRAF2 restricts the ability of TNFR1 to induce caspase-8 activation and apoptosis [144,145,146]. Notably, TNFR2 and other TRAF2-interacting TNFRs, particularly Fn14, not only engage the alternative NFκB pathway but also sensitize for TNFR1-induced cell death by lowering the TNFR1-accessible pool of TRAF2 and cIAPs [134,135,145,147,148,149,150,151,152]. Intriguingly, TRAF2 is not only recruited to the TNFR1 signaling complex within seconds to few minutes but can also be part of TNFR1-induced delayed formed cytosolic complexes enabling caspase-8 activation and apoptosis (complex IIa) or RIPK1 phosphorylation and necroptosis (complex IIb). Inhibition or depletion of cIAPs largely mirrors the effects of TRAF2 depletion on TNFR1 signaling. Therefore, it is tempting to speculate that a significant part of the effects of TRAF2 on TNFR1 signaling is based on its ability to recruit the cIAPs to the TNFR1 signaling complex and to the cytosolic caspase-8/RIPK1-containing complexes IIa and IIb. While TRAF2 molecules associated with TNFR1 seem to be sufficient to fulfill the function of TRAF2 as a transducer of TNFR1-induced classical NFκB signaling, TRAF2 associated with complex IIa and IIb appears to act as an inhibitor of caspase-8 and RIPK1 kinase activation. The NFκB-stimulating activity of TNFR1 signaling complex-associated TRAF2 has been attributed to the K63-ubiquitination of several TNFR1 signaling complex proteins, particularly RIPK1, by the TRAF2-associated cIAPs enabling efficient recruitment of the LUBAC, the TAB2-TAK1 complex and the IKK complex (see also Section 2.1. above).
Initially, the antiapoptotic activity of TRAF2 was attributed to its relevance for activation of the classical NFκB pathway which results in upregulation of various antiapoptotic proteins, including cFLIP, cIAP2, B-cell lymphoma 2 (Bcl-2) and Bcl-xL, and many more [144,145,153,154,155]. However, a key observation suggested that TRAF2 has antiapoptotic activity, at least in TNFR1 signaling, independent from induction of NFκB-regulated antiapoptotic factors. When cells were primed for a few hours via TNFR2, TRAF2-associated NFκB signaling-promoting functions in the TNFR1 signaling complex, such as RIPK1 ubiquitination and IKK recruitment, were severely affected [151]. In contrast, when TNFR1 and TNFR2 are costimulated, TNFR2-induced depletion of TRAF2 which requires 1-3 h to become apparently sensitized for TNFR1-induced apoptosis occurring with similar slow kinetics but fails now to inhibit the activity and assembly of the rapidly formed classical NFκB-stimulating TNFR1 signaling complex [134,151]. How TRAF2 inhibits TNFR1-induced caspase-8 maturation, which takes place in complex IIa in the latter NFκB-independent scenario, is not fully understood. A part of the explanation could be that TRAF2 K48-ubiquitinates caspase-8 to promote proteasomal degradation of the p43 and p18 fragments of caspase-8. These fragments are generated during death receptor-induced processing of procaspase-8 to mature heterotetrameric caspase-8, and their TRAF2-mediated degradation has been described in context of TRAIL death receptor signaling as a way to downregulate TRAIL sensitivity [114,115]. Worth mentioning, the E3 ligases cIAP1 and cIAP2 are dispensable for TRAF2-mediated ubiquitination of caspase-8 and instead it seems that TRAF2 itself acts here as an E3 ligase by virtue of its RING domain [114]. Death receptor-induced caspase-8 activation normally inhibits DR-induced necroptosis by cleavage of RIPK1 (and possibly other caspase-8 substrates) so that there is no induction of necroptotic cell death [156]. However, when caspase-8 is inhibited, e.g., by drugs or pathogen-encoded proteins, this strongly proinflammatory form of programmed cell death occurs. Interestingly, although TRAF2 acts as an inhibitor of the necroptosis inhibitor caspase-8 as just described, it has been discovered that TRAF2 also protects from DR-induced necroptosis [157,158]. Thus, it appears that the antinecroptotic activity of TRAF2 in DR signaling overrides its potential pronecroptotic activity resulting from caspase-8 inhibition. The antinecroptotic activity has been again traced back to K63 ubiquitination of RIPK1, however, yet in context of complex IIa and/or the RIPK1-RIPK3 necrosome. Sequestration of TRAF2 to liganded TRAF-interacting TNFRs, such as Fn14, consequently results in enhancement of death receptor- and TLR3-induced necroptosis, too [157,159]. The in vivo relevance of the cell death-sensitizing activity of TRAF2/cIAP sequestration by TRAF2-interacting TNFRs is poorly understood. Animal studies suggest that this mechanism is responsible for the high sensitivity of intestinal epithelial cells for TNF-induced killing but could also be operative in the cell death occurring after acute kidney injury or to pathogen-induced hyperinflammation in patients suffering on X-linked inhibitor of apoptosis (XIAP) deficiency [160,161,162,163]. Antiapoptotic activities of TRAF2 have also been described in scenarios beyond death receptor signaling, for example in UV- and oxidative stress-induced apoptosis [155,164].
Apoptosis induction by death receptors but also by drugs or other stressors of cellular homeostasis (see also below Section 3.2) can imply generation of reactive oxygen species (ROS) and sustained activation of the cJun N-terminal kinase (JNK) pathway as an enhancing or even essential mechanism [165]. Many of the triggers of apoptotic JNK signaling engage the JNK pathway by TRAF2-dependent activation of the MAP3K apoptosis signal-regulating kinase 1 (ASK1) (e.g., [166,167,168,169]). The latter is kept inactive in cells by binding to thioredoxin (Trx). ROS formation, e.g., in response to TNF, results in dissociation of the ASK1-Trx complex giving TRAF2 access to ASK1 [170]. The central role of the TRAF2-ASK1 interaction for ASK1-driven JNK signaling is also reflected by the fact that the TRAF2-ASK1 interaction is controlled/modulated by various proteins, such as ASK1-interacting protein 1 (AIP1), which enhances TRAF2-ASK1 complex formation, calcium and integrin-binding protein 1 (CIB1), which interferes with TRAF2-ASK1 interaction [171] and the protein arginine methyltransferases-1 (PRMT1), which modifies ASK1 and stabilizes its association with Trx [172]. Worth mentioning, TRAF2 has also been found to bind and stimulate the mammalian ste20-like kinase 1 (MST1) which is also activated by reactive oxygen species and stimulate apoptotic JNK signaling [79,173]. Moreover, TRAF2-MST1 interaction and MST1 activation occur downstream of the ROS-induced dissociation of an inactive Trx-MST1 complex [79]. However, whether and how TRAF2, MST1, and ASK1 act together is currently unclear.
3.2. TRAF2 in ER Stress and Autophagy
The unfolded protein response (UPR) occurs in reaction to ER stress due to accumulation of misfolded proteins. If ER stress is moderate the UPR protect cells by triggering the production of factors (e.g., chaperones) helping to restore fidelity of protein folding, to maintain general functionality and survival. In situations of chronic and strong ER stress, however, the UPR can also trigger cell death programs [174,175]. There are three major sensor proteins for ER stress: (i) activating transcription factor-6 (ATF6), a ER residing transmembrane protein with a cytosolic transcription factor domain which can be released by proteolytic processing, (ii) protein kinase RNA-like ER kinase (PERK), which reduces general protein synthesis by phosphorylation of eukaryotic initiation factor 2alpha (eIF2α) but also promotes enhanced translation of selected mRNAs including that encoding the transcription factor ATF4 and (iii) the bifunctional inositol-requiring enzyme 1alpha [IRE1α, also named ER to nucleus signaling 1 (ERN1)] which has serine/threonine-protein kinase and RNAse activity. Upon sensing ER stress by yet poorly understood mechanisms (dissociation from the chaperone GRP-78/BiP and/or direct binding of misfolded proteins), IRE1α dimerizes and becomes activated by trans-autophosphorylation. This stimulates the RNAse activity of IRE1α and enables the recruitment of TRAF2 [174,176] (Figure 4). With the help of its RNAse activity, IRE1α splices out in a spliceosome-independent manner a small intron from the mRNA of the transcription factor XBP1, eventually resulting in a switch from the production of an inactive “unspliced” form (XBP1u) to an active transcription factor (XBP1s) but also cleaves various RNAs resulting in IRE1-dependent decay of mRNA (RIDD) [177]. Interaction of TRAF2 with phosphorylated dimerized IRE1α leads to the recruitment and activation of ASK1 and stimulation of MAP kinase signaling pathways resulting in the engagement of p38, extracellular-regulated kinase (ERK) but particularly of JNKs (Figure 4). However, the IRE1α/TRAF2 dyad can also promote activation of the classical NFκB pathway or cell death programs (Figure 4).
Figure 4.
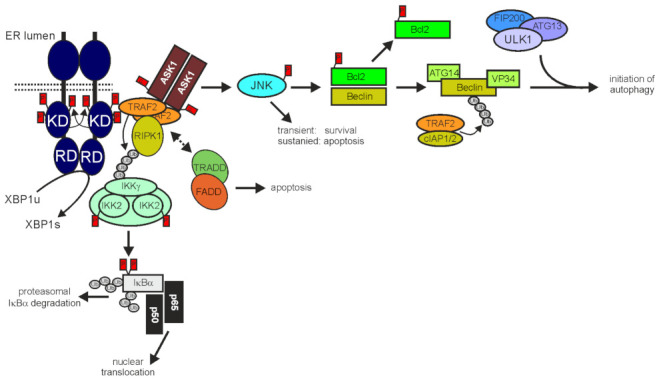
TRAF2 in IER1 signaling. After ER stress-induced dimerization IREα triggers production of the transcription factor XBP1 by its endonuclease activity engages splicing and recruits TRAF2 to trigger ASK1/JNK signaling but also, by still poorly studied mechanisms, classical NFκB signaling and apoptosis. The IRE1α/TRAF2/ASK/JNK axis is also connected with autophagy by the JNK-mediated phosphorylation of the Beclin inhibitory Bcl-2 protein. Interestingly, TRAF2 in concert with cIAP1/2 can further contribute to autophagy by K63-ubiquitination of Beclin. For more details refer to main text.
IRE1α-induced TRAF2-mediated NFκB activation involves K63-ubiquitination of RIPK1 [178]. Furthermore, it has been discovered that the IRE1α/TRAF2/ASK1 complex recruits TRADD and FADD in response to ER stress [179]. The recruitment of these DD-containing proteins resembles the situation in death receptor-signaling but this similarity has yet not evaluated in molecular detail. In an early phase, IRE1α/TRAF2-mediated JNK signaling contributes to cell survival by inducing transcription of cIAP1, cIAP2, and xIAP [180]. Sustained JNK signaling, however, can then become proapoptotic by activation of the proapoptotic Bcl-2 family members BIM and BID and inhibition of antiapoptotic Bcl-2 family members, such as Bcl-2, Bcl-xL and MCL1 [174]. It is worth mentioning that the proapoptotic IRE1α/TRAF2/ASK1/JNK axis might cooperate with the PERK/ATF4-dependent induction of TRAIL death receptors [174,181] and/or the IRE1α/TRAF2/IKK-induced production of TNF [182]. TRAF2-deficient MEFs are more susceptible for ER stress-induced apoptosis than wild-type MEFs [183] but whether this is due to the role of TRAF2 in ER stress/IRE1α signaling or rather reflect defects in other TRAF2-dependent activities affecting the general apoptosis sensitivity of cells is unclear. The IRE1α/TRAF2/ASK1/JNK axis is also connected with autophagy as discussed below.
Autophagy, which occurs in various forms, such as macroautophagy, microautophagy, chaperone-mediated autophagy, or selective autophagy for certain proteins, is a cellular housekeeping activity to purge intracellular “waste”, e.g., protein aggregates, damaged organelles and intracellular pathogens, but is also important for maintenance of plasma membrane integrity [184,185,186,187]. In view of these activities, it is not surprising that steady-state autophagy is increased when cellular homeostasis is disturbed, e.g., by nutrient deficiency, genomic instability or infection, but also by ER stress and cell death programs. Since TRAF2 plays a role in IRE1α-signaling, regulation of cell death and the NFκB system, it can also be of relevance for autophagy. Indeed, TRAF2 and TRAF2-associated proteins have been implicated in several ways in the crosstalk between autophagy, ER stress, cell death and inflammation, particularly in context of cancer development and cancer treatment.
Bcl-2 and Bcl-xL attenuate autophagy by binding and inhibition of Beclin-1, thereby hindering the latter to stimulate the ULK1 complex, which promotes autophagosome initiating vesicle nucleation [188]. Accordingly, BH3-only proteins competing with Beclin for Bcl-2 binding and JNK-mediated phosphorylation of Bcl-2, resulting in reduced Beclin-1/Bcl-2 interaction, which are able to enhance autophagy [189,190]. Therefore, apoptosis inducers engaging these mechanisms can have a stimulatory effect on autophagy that typically results in reduced cell death [189]. For example, apoptosis-reducing TRAF2- and JNK-mediated stimulation of autophagy have been demonstrated for TRAIL and the IRE1α inhibitor protein Bax inhibitor-1 [191,192,193]. TRAF2 cannot only promote Beclin activation due to its role in JNK stimulating signaling pathways but in concert with cIAP1 and cIAP2 and/or sphingosin kinase 1, and also directly by K63-polyubiquitination of Beclin [43,194]. Worth mentioning, JNK can also inhibit context-dependent autophagy flux. For example, nitrobenzoxadiazole derivatives which antagonizes sequestration of TRAF2 by glutathione transferase and, which are under consideration as anticancer drugs, impair clearance of autophagosomes in a JNK-dependent manner [195].
TRAF2 and cIAP1 have also been found to mediate PTEN-induced kinase 1 (PINK1)/Parkin-independent mitophagy in response to the anti-parasitic lactone ivermectin by promoting ubiquitination and fragmentation of mitochondria [119]. Similarly, the anti-inflammatory triterpene celastrol binds to Nur77, resulting in enhanced TRAF2-Nur77 interaction and eventually mitophagy [85]. In contrast, to ivermectin-induced TRAF2-dependent mitophagy and celastrol-induced TRAF2-Nur77-mediated mitophagy seems to involve PINK1 and Parkin [85]. Indeed, it has been discovered i) that TRAF2 interacts with Parkin in mitophagy induced by the mitochondria-depolarizing compound carbonyl cyanide m-chlorophenyl hydrazine and ii) that TRAF2 partly substitutes Parkin as an E3 ligase in this scenario [118]. Therefore, TRAF2 could contribute to mitophagy in different ways.
4. TRAF2 and the NFκB System in Oncogenesis
4.1. TRAF2 in EBV-associated Oncogenesis
Soon after TRAF2 and TRAF1 were described the first time, there was evidence that TRAF2 can also play a role in tumor biology. Both molecules, and later also TRAF3, TRAF5, and TRAF6, were recognized as part of the plasma membrane-associated protein complex instructed by the latent infection membrane protein 1 (LMP1) of Epstein-Barr virus (EBV, human γ -herpesvirus 4) [35,36,196]. Several lymphoproliferative diseases, including malignant ones, are associated with EBV infection, such as Burkitt lymphoma and Hodgkin’s lymphoma, but also non-lymphoid malignancies, particularly nasopharyngeal carcinoma (NPC) and gastric cancer [197]. LMP1 is frequently found in EBV-associated cancers and acts as an oncogene by stimulating various signaling pathways, including those resulting in the activation of NFκB transcription factors and MAP kinases such as JNK, p38, and ERK. In accordance with a central role of the NFκB system in EBV/LMP1-dependent oncogenesis, genomic analysis of NPC revealed in LMP1-independent cases somatic aberrations resulting in constitutive NFκB activation [198]. TRAF2 in addition to TRAF1, TRAF3, TRAF5, and TRAF6 recruit to a PXQXT/S motif in a plasma membrane-proximal domain of the cytoplasmic tail of LMP1 [199,200,201]. This domain has been designated as transformation effector site 1 (TES1) or C-terminal activation region 1 (CTAR1) and is needed, together with a more C-terminally located domain called TES2/CTAR2, for transformation of B-lymphocytes [202,203,204]. TES1/CTAR1 and TES2/CTAR2 cooperate in LMP1-induced TRAF protein-mediated activation of NFκB transcription factors, JNK and p38 [130,203,204]). It is worth mentioning that TES2/CTAR2 recruits TRADD and BS69 and that there is initial evidence that these proteins in turn recruit TRAF2 and TRAF6 to the CTAR2, as well [203,204]. Studies in TRAF2-deficient B-cell lines failed to demonstrate a crucial role of TRAF2 in LMP1 signaling [130,205] while there is clear evidence for the relevance of other TRAF proteins, particularly TRAF3 and TRAF6, in this respect [201,205,206]. Likewise, there were no effects of TRAF2-TRAF5 double deficiency on LMP1-induced nuclear translocation of RelA in embryonal fibroblasts [206]. However, in TRAF2-deficient models there is already considerable constitutive p100 processing, thus constitutive activation of the alternative NFκB pathway. It is therefore challenging in these cell models to draw conclusions on the relevance of the LMP1-TRAF2 interaction for LMP1-induced alternative NFκB signaling.
Since LMP1 aggregates in the plasma membrane and recruits TRAF molecules, it obviously mimics in several, but not all, aspects the ligand-induced signaling complexes of receptors of the TNFRSF. Indeed, activation of both NFκB signaling pathways and JNKs in response to EBV infection has been attributed to LMP1 signaling via TRAF2 and TRAF1 (but also TRAF6), worth mentioning without the involvement cIAP1 and cIAP2 [199,207,208,209]. LMP1-induced NFκB-mediated upregulation of A20 and TRAF1 and subsequent recruitment of these factors along with the LUBAC is another aspect where LMP1 signaling resembles that of TRAF2-interacting TNFRs [36,209,210,211]. Indeed, a genome-wide siRNA screen for proteins involved in LMP1-induced NFκB signaling resulted in the identification of 155 proteins, of which 79 was similarly relevant for TNF-induced NFκB activation [212]. With respect to its activities in B-cells and its ability to recruit TRAF1, TRAF2, TRAF3, TRAF5, and TRAF6, LMP1 largely mimics activated CD40. The general similarities between the signaling complexes of LMP1 and TNFRs, particularly CD40, however, should not hide the fact that there are clear differences. For example, as mentioned above, LMP1 signaling was found to be normal in TRAF2-deficient B-cell lines while CD40 signaling was reduced in the same cell lines and in B-cells of mice with TRAF2-deficient B-cells [130,205,213]. Vice versa, CD40 singling was unaffected in TRAF3-deficient B-cell lines while LMP1 signaling was strongly attenuated [130,205]. Furthermore, CD40, but not a CD40 chimera with the C-terminal cytosolic tail of LMP1, triggers TRAF2-dependent degradation of TRAF3 [214,215]. These differences have been traced back to the higher efficiency with which CD40 recruits TRAF2 [216] and illustrate that not only the ensemble of TRAF proteins interacting with a certain receptor determines the signaling output but also the absolute and relative strengths of the receptor-TRAF protein interactions.
LMP1 is not the only EBV protein interacting with TRAF2. The EBV protein Na, which is crucially involved in the reactivation of EBV in latent infected cells leading to cell lysis, also interacts with TRAF2 [217,218]. Na utilizes TRAF2 to engage JNK-dependent expression of lytic gene expression [218]. It is worth mentioning that Na overexpression, similar to LMP1, also stimulates alternative but not classical NFκB signaling [218]. This again emphasizes that TRAF2-dependent processes, here TRAF2-Na or LMP1-Na interaction and TRAF2-dependent NIK degradation, might compete for TRAF2 resulting in hardly predictable activities of TRAF2-dependent events.
4.2. TRAF2 and the Alternative NFκB Pathway in Multiple Myeloma and B-cell Lymphoma
Activation of the NFκB system has been recognized in a variety of tumor types, however, whether this correlates with genetic mutations was poorly investigated for a long time. Gene expression profiling data and functional studies revealed that development of multiple myeloma (MM) is frequently associated with mutations resulting in enhanced NFκB signaling particularly via the alternative NFκB pathway [219,220,221,222]. Most of these mutations are found in the gene of TRAF3 but mutations negatively affecting expression or function of TRAF2 were also frequently found defining TRAF2 (and TRAF3) in MM as a tumor suppressor. Mouse models with deletion of cIAP1 and cIAP2 but not of either protein alone makes the survival of B-cells independent of BaffR signaling and led to their uncontrolled accumulation in vivo, resulting in B-cell lineage malignancies [223]. Thus, as so often the activity of TRAF2 and TRAF3, here as tumor suppressors, require support by cIAP1 and cIAP2. Furthermore, a Crispr/Cas9 genetic screen identified TRAF2 as factor promoting antitumoral IMID activity via activation of alternative NFκB and ERK signaling [224]. Recurrently occurring mutations in TRAF2 were also observed in mantle cell lymphoma resistant against the B-cell receptor (BCR) signaling inhibitor ibrutinib [225].
Constitutive activation of the NFκB system is also a hallmark of diffuse large B-cell lymphoma (DLBCL) particularly of the activated B-cell-like subtype (ABC-DLBCL). In fact, somatic mutations in various genes encoding components of the NFκB system have been identified including mutations in the TRAF2 gene [226,227]. These studies argue for an antitumoral role of TRAF2 in DLBCL, presumably due to its ability to suppress the alternative NFκB pathway. However, there is evidence that TRAF2 can also elicit protumoral activity in DLBCL. Immunohistochemical analyses revealed strong expression of TRAF2 in ABC-like DLBCL with significant association with reduced progression-free survival [228]. Moreover, functional genetic screens in DLBCL cell lines identified TRAF2 as a factor conferring resistance against mucosa-associated lymphoid tissue lymphoma translocation protein 1 (MALT1) inhibitor and the cereblon E3 ligase–modulating agent CC-122 [229,230]. One of the somatic mutations in TRAF2 identified in DLBCL results in enhanced TRAF2-dependent classical NFκB activation [226]. In view of the general relevance of the NFκB system for the physiology and pathophysiology of B-cells, it is tempting to speculate that the contradictory impact of TRAF2 on DLBCL reflects its opposing effects on the classical and alternative NFκB pathway.
In accordance with the notion that the alternative NFκB pathway and therefore also TRAF2 and TRAF3 act as tumor suppressors in B-cells, mice with B-cells deficient in TRAF3 expression demonstrate prolonged B-cell survival and develop spontaneous B-cell lymphoma at a higher age [231,232]. Likewise, transgenic mice expressing Bcl2 and a RING/Zn finger domain-deletion mutant of TRAF2 develop lymphoma with high frequency [233]. Worth mentioning, the TRAF2 deletion mutant expressed in these mice causes degradation of endogenous TRAF2 but also its own degradation, suggesting that it mimics TRAF2 deficiency [234].
4.3. TRAF2 and Wnt/β-catenin Signaling
TRAF2 mutations have also been described for colon cancer [235]. Furthermore, TRAF2 transcript expression is much higher in colorectal cancer compared to benign tissues and is negatively associated with patient survival [46]. A dominant oncogenic driver of colon cancer development is the Wnt/β-catenin signaling pathway culminating in the formation and activation of a nuclear transcriptional complex containing β-catenin, T-cell factor 4 (TCF4) and the TRAF2- and Nck-interacting kinase (TNIK). TRAF2 is not only able to interact with the latter [105] but also binds β-catenin [46]. More important, TRAF2 binds and stabilizes the β-catenin-TNIK-TCF4 complex thereby crucially contributing to TCF4 activation [46]. There is also initial evidence that TRAF2 favor colon cancer development beyond its role in the Wnt/β-catenin. Peng at al [92] could indicate that TRAF2 contributes to EGF-induced ribosomal S6 kinase 2 (RSK2) activation in colon cancer cell lines and Jia et al. [72] reported recently that TRAF2 binds LRPPRC to ubiquitinate argininosuccinate synthase 1 marking this enzyme for degradation resulting in reduced arginine synthesis and the latter is required for tumor growth. Colon cancer is possibly not the only tumor entity where the β-catenin-TRAF2 axis gains relevance. For chronic myelogenous leukemia (CML), it has been reported that the TRAF2-interacting TNFRSF receptor CD27 stimulates the oncogenic Wnt-β-catenin-TNIK-TCF4 pathway, resulting in enhanced proliferation of leukemia stem cells and leukemia progression in a TRAF2-dependent manner [236].
4.4. TRAF2 in Breast Cancer and Other Solid Tumors
The NFκB system in general affects a variety of cancer relevant processes, and it is thus not surprising that TRAF2 via its role in NFκB signaling not only contributes to malignant transformation of lymphoma but also to development of solid cancers. For example, in breast cancer there is ample evidence that NFκB signaling promotes tumor progression, metastasis and resistance against chemo- and radiotherapy ([237,238]). Perhaps most intriguing is the role of the TRAF2-NFκB connection in breast cancer cell transformation by the breast cancer oncogene IκB kinase ε (IKKε; IKKi). IKKε expression is upregulated in >30% of breast cancers and to an even higher frequency in glioblastoma (50%) and pancreatic ductal adenocarcinoma (65%) [239]. IKKε stimulates the K63-ubiquitination activity of the TRAF2-cIAP1 and TRAF2-cIAP2 complexes by phosphorylation of serine 11 on TRAF2 resulting in NFκB activation and various protumoral effects in vitro and in vivo, such as increased cell proliferation, anchorage-independent colony formation, and enhanced tumor formation [66,240]. Protumoral TRAF2 activity in breast carcinoma is also evident from xenogeneic tumor models with human MDA-MB-231 breast cancer cell variants overexpressing TRAF2, which indicated enhanced orthotopic tumor growth in mice after injection into the mammary fat pad and increased skeletal tumors after intra-tibial application [241]. Interestingly, it has been reported that receptor activator of NFκB (RANK)-c, a splice form of the TRAF2-interacting TNFR RANK, which is found in 3.2% of breast cancer patients and which acts in a dominant-negative fashion on RANK-induced NFκB activation, is inversely correlated with disease grade [242,243]. Moreover, RANK-c requires its TRAF2 binding sites to elicit its tumor attenuating NFκB-inhibitory activity pointing to TRAF2 sequestration as mode of action. Furthermore, there is evidence that certain microRNAs (miR-892b, miR-502-5p, miR-205-5p) downregulate TRAF2 expression and thereby suppress breast cancer development [244,245,246]. Similarly, the E3 ligase carboxyl terminus of Hsp70-interacting protein (CHIP), which triggers TRAF2 degradation, has tumor suppressive activity in breast and gastric cancer cells [48,247,248]. Furthermore, a crucial role of the TRAF2-NFκB axis as an adaptive survival mechanism in response to EGFR oncogene inhibition has been reported for lung cancer and there is also evidence that TRAF2 expression confers resistance against irradiation [52,249]. In accordance with the antiapoptotic activities of TRAF2, it has been discovered in various solid cancer cell lines that TRAF2 expression via NFκB activation protects against radio- and chemotherapy but a detailed discussion of this issue is behind the scope of this review.
5. TRAF2 and the Response to Immune Checkpoint Blockade
The approval of immune checkpoint inhibitors (ICIs) brought the clinical breakthrough for immunotherapy. Despite the often-impressive clinical efficacy of immune checkpoint blockade (ICB), however, many patients do not respond or develop dose-limiting autoimmune effects. Therefore, there are considerable ongoing efforts to identify drugs that enhance clinical efficacy of ICB. Two comprehensive independent studies, utilizing different methodologies, argue for TRAF2 as a novel and promising target whose inhibition could synergize with ICIs. Vredevoogd et al. [250] identified in a genome-wide Crispr-Cas9 screen in IFNγ-resistant melanoma cells TRAF2 and cIAP1 as the two major hits increasing the sensitivity for IFNγ-independent killing by CD8+ T-cells. In view of the relevance of TRAF2 and the cIAPs to protect from TNFR1-induced cell death (see Section 3.1), this suggested that the sensitivity of tumor cells for TNF cytotoxicity is a crucial factor for ICB efficacy. Aligned with this idea, there were also hits in other factors regulating TNF cytotoxicity. Furthermore, while the mutational status of the TNF system in untreated tumors failed to correlate with patient survival, a positive correlation between TNF expression and the response to anti-PD1 therapy has been observed [250]. Follow-up in vitro and in vivo experiments indeed indicated that TRAF2 deficiency sensitizes tumor cells for killing by TNF expressed by CD8+ T cells. Notably, it has been reported in this study, too, that TNF-like weak inducer of apoptosis (TWEAK), the ligand of Fn14, also sensitizes cancer cells for TNF-mediated killing by CD8+ T cells. This corresponds very well to the already discussed ability of Fn14 to deplete the cytosolic pool of TRAF2-cIAP1/2 complexes, (see Section 3.1). In a second approach whole-exome and transcriptomic data along with clinical outcome data derived of more than >1000 patients treated with ICIs were evaluated in a meta-analysis for predictors of ICI response. Worth mentioning, copy-number analysis in this study also identified loss of 9q34, the position of the TRAF2 gene, to be positively associated with clinical response [251].
6. Therapeutic TRAF2 Targeting Strategies
In view of its often protumoral activities and its ICB antagonizing effects, TRAF2 is an obvious potential target for tumor therapy. However, since TRAF2 utilizes its pleiotropic functions by a variety of binding partners, which interact with different parts of the molecule, it is difficult to define a side in TRAF2 enabling general inhibition of TRAF2. Since some TNFRs deplete cytosolic TRAF2 pools and even trigger its subsequent degradation, agonists of such receptors could be considered as a kind of TRAF2 inhibitor, at least as inhibitor of cytosolic TRAF2 functions. This perception is interesting for targeting the ICB antagonizing effects of TRAF2. Indeed, as discussed above, initial studies indicated that TWEAK acts as an inhibitor of the ICB antagonizing TRAF2 activity [250]. Eventually, agonists of TRAF2-interacting receptors act here as “selective” inhibitors of TRAF2 survival functions (or other cytosolic TRAF2 activities) but not as general TRAF2 inhibitors. Indeed, the TNFRs may even actively exploit TRAF2 to exert proinflammatory activity. Since TRAF2 fulfills many of its activities with essential support of cIAP1 or cIAP2, inhibitors of these molecules can also be considered to be TRAF2 inhibitors. This appears particularly interesting because a variety of cIAP antagonists (also called SMAC mimetics) are under preclinical and clinical evaluation for cancer therapy [252]. The feasibility of the consideration of agonists of cytosolic TRAF2-depleting TNFRs, such as Fn14, and IAP antagonists as pseudo TRAF2 inhibitors, is underscored by the fact that TRAF2 deficiency, Fn14 agonists and IAP antagonists trigger the same cellular hallmarks, namely activation of the alternative NFκB pathway and sensitization for TNF-induced cell death. Nevertheless, TNFR agonists can trigger TRAF2-dependent and independent signaling pathways and IAPs have TRAF2-independent activities, too. Thus, direct TRAF2 inhibitors still have the potential to elicit therapeutic activity beyond TNFR agonists and IAP antagonists.
Liquidambaric acid (LDA, or betulonic acid) is a pentacyclic triterpenoid which has been decades under investigation as an anti-cancer compound [253]. LDA has just been recently identified as a first TRAF2-binding small molecule. LDA prevents β-catenin-TRAF2 interaction and inhibits the Wnt/β-catenin pathway and colon cancer development in a xenogeneic colon cancer model [46]. Interestingly, LDA inhibited the interaction of TRAF2 and β-catenin but demonstrated no effect on TRADD binding of TRAF2 [46]. This example illustrates that it could be possible to develop low molecular weight inhibitors, which specifically antagonize certain aspects of TRAF2 biology.
IAP antagonists demonstrate low toxicity, are well tolerated and displayed good antitumor activity in preclinical models. However, in clinical trials with IAP antagonists as single agents, only limited therapeutic activity has been observed. Because of the intersection of effects of IAP antagonists and TRAF2 deficiency, it appears plausible that TRAF2 inhibitors, similarly to IAP antagonists, may only demonstrate limited activity in patients. It could be therefore necessary to combine such compounds with other drugs to exploit the possible antitumoral potential of TRAF2 inhibition for cancer therapy. Against the background that TRAF2 and cIAPs ([250,254]) protect tumor cells against the cytotoxic action of CD8+ T-cells and NK cells after ICB, combination therapies of TRAF2 inhibitors and checkpoint inhibitors appears particularly interesting. Clinical trials with IAP antagonists and ICIs are ongoing [252] and will provide first hints in this direction.
7. Conclusions
A major general conclusion that can been drawn from the available TRAF2 literature is that TRAF2 is part of a large variety of constitutively formed and signal-induced protein complexes that act in quite different cellular processes and in different cellular compartments. Importantly, there is evidence that these TRAF2-dependent processes compete for a limited pool of TRAF2 (and TRAF2-associated factors) resulting in complex context-dependent crosstalk mechanisms. A consequence of increased TRAF2 expression, as often observed in tumor cells, is that competition for TRAF2 loses functional relevance. Therefore, increased TRAF2 expression may not only generally enhance TRAF2-dependent processes but also weaken crosstalk mechanisms that reciprocally counterbalance TRAF2-dependent processes in non-transformed cells. For example, increased TRAF2 expression may not only increase the ability of TRAF2-interacting TNFRs to engage the classical NFκB pathway but will also limit their capacity to sensitize for cell death by TRAF2-cIAP1 sequestration. Thus, deregulated TRAF2 expression could not only change the amplitude of TRAF2-dependent responses but also the qualitative outcome of the whole network of TRAF2-mediated processes.
A second conclusion is that TRAF2, despite its function as a tumor suppressor in B-cells, is a promising target for tumor therapy, particularly in combination with checkpoint inhibitors. An important and possibly decisive question in this context is whether effective inhibition of TRAF2 can be achieved. Indirect inhibition of TRAF2, e.g., with agonists of TRAF2-depleting receptors, in addition to direct inhibition of TRAF2 by small molecules appears conceivable. Both possibilities have been limitedly investigated so far and define a major challenge in the field in the next years. However, the clinical development of TRAF2 inhibitors should be accompanied by close monitoring for signs of autoimmunity or B-cell hyperplasia.
Author Contributions
Conceptualization, D.S. and H.W.; writing—review and editing, D.S., J.W. and H.W.; visualization, J.W. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Funding Statement
This work was supported by grants of the Deutsche Forschungsgemeinschaft to D.S. (project number Si 1128/6-1) and Deutsche Krebshilfe to H.W. (project number 70114009).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rothe M., Pan M.G., Henzel W.J., Ayres T.M., Goeddel D.V. The TNFR2-TRAF signaling complex contains two novel proteins related to baculoviral inhibitor of apoptosis proteins. Cell. 1995;83:1243–1252. doi: 10.1016/0092-8674(95)90149-3. [DOI] [PubMed] [Google Scholar]
- 2.Rothe M., Wong S.C., Henzel W.J., Goeddel D.V. A novel family of putative signal transducers associated with the cytoplasmic domain of the 75 kDa tumor necrosis factor receptor. Cell. 1994;78:681–692. doi: 10.1016/0092-8674(94)90532-0. [DOI] [PubMed] [Google Scholar]
- 3.Wajant H., Grell M., Scheurich P. TNF receptor associated factors in cytokine signaling. Cytokine Growth Factor Rev. 1999;10:15–26. doi: 10.1016/S1359-6101(98)00023-9. [DOI] [PubMed] [Google Scholar]
- 4.Vince J.E., Pantaki D., Feltham R., Mace P.D., Cordier S.M., Schmukle A.C., Davidson A.J., Callus B.A., Wong W.W., Gentle I.E., et al. TRAF2 must bind to cellular inhibitors of apoptosis for tumor necrosis factor (tnf) to efficiently activate nf-{kappa}b and to prevent tnf-induced apoptosis. J. Biol. Chem. 2009;284:35906–35915. doi: 10.1074/jbc.M109.072256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Workman L.M., Zhang L., Fan Y., Zhang W., Habelhah H. TRAF2 Ser-11 Phosphorylation Promotes Cytosolic Translocation of the CD40 Complex To Regulate Downstream Signaling Pathways. Mol. Cell Biol. 2020;40:e00429-19. doi: 10.1128/MCB.00429-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou W., Yao J., Wang G., Chen Z., Li Z., Feng D., Li Y., Qasim W., Tan W., Ning S., et al. PKCζ phosphorylates TRAF2 to protect against intestinal ischemia-reperfusion-induced injury. Cell Death Dis. 2017;8:e2935. doi: 10.1038/cddis.2017.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas G.S., Zhang L., Blackwell K., Habelhah H. Phosphorylation of TRAF2 within its RING domain inhibits stress-induced cell death by promoting IKK and suppressing JNK activation. Cancer Res. 2009;69:3665–3672. doi: 10.1158/0008-5472.CAN-08-4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qian Y., Wang Z., Lin H., Lei T., Zhou Z., Huang W., Wu X., Zuo L., Wu J., Liu Y., et al. TRIM47 is a novel endothelial activation factor that aggravates lipopolysaccharide-induced acute lung injury in mice via K63-linked ubiquitination of TRAF2. Signal. Transduct. Target. Ther. 2022;7:148. doi: 10.1038/s41392-022-00953-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li S., Wang L., Dorf M.E. PKC phosphorylation of TRAF2 mediates IKKalpha/beta recruitment and K63-linked polyubiquitination. Mol. Cell. 2009;33:30–42. doi: 10.1016/j.molcel.2008.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li S., Wang L., Berman M.A., Zhang Y., Dorf M.E. RNAi screen in mouse astrocytes identifies phosphatases that regulate NF-kappaB signaling. Mol. Cell. 2006;24:497–509. doi: 10.1016/j.molcel.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim Y.S., Nedospasov S.A., Liu Z.G. TRAF2 plays a key, nonredundant role in LIGHT-lymphotoxin beta receptor signaling. Mol. Cell Biol. 2005;25:2130–2137. doi: 10.1128/MCB.25.6.2130-2137.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arch R.H., Thompson C.B. 4-1BB and Ox40 are members of a tumor necrosis factor (TNF)-nerve growth factor receptor subfamily that bind TNF receptor-associated factors and activate nuclear factor kappaB. Mol. Cell Biol. 1998;18:558–565. doi: 10.1128/MCB.18.1.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawamata S., Hori T., Imura A., Takaori-Kondo A., Uchiyama T. Activation of OX40 signal transduction pathways leads to tumor necrosis factor receptor-associated factor (TRAF) 2- and TRAF5-mediated NF-kappaB activation. J. Biol. Chem. 1998;273:5808–5814. doi: 10.1074/jbc.273.10.5808. [DOI] [PubMed] [Google Scholar]
- 14.Rothe M., Sarma V., Dixit V.M., Goeddel D.V. TRAF2-mediated activation of NF-kappa B by TNF receptor 2 and CD40. Science. 1995;269:1424–1427. doi: 10.1126/science.7544915. [DOI] [PubMed] [Google Scholar]
- 15.Akiba H., Nakano H., Nishinaka S., Shindo M., Kobata T., Atsuta M., Morimoto C., Ware C.F., Malinin N.L., Wallach D., et al. CD27, a member of the tumor necrosis factor receptor superfamily, activates NF-kappaB and stress-activated protein kinase/c-Jun N-terminal kinase via TRAF2, TRAF5, and NF-kappaB-inducing kinase. J. Biol. Chem. 1998;273:13353–13358. doi: 10.1074/jbc.273.21.13353. [DOI] [PubMed] [Google Scholar]
- 16.Gravestein L.A., Amsen D., Boes M., Calvo C.R., Kruisbeek A.M., Borst J. The TNF receptor family member CD27 signals to Jun N-terminal kinase via Traf-2. Eur J. Immunol. 1998;28:2208–2216. doi: 10.1002/(SICI)1521-4141(199807)28:07<2208::AID-IMMU2208>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto H., Kishimoto T., Minamoto S. NF-kappaB activation in CD27 signaling: Involvement of TNF receptor-associated factors in its signaling and identification of functional region of CD27. J. Immunol. 1998;161:4753–4759. [PubMed] [Google Scholar]
- 18.Gedrich R.W., Gilfillan M.C., Duckett C.S., Van Dongen J.L., Thompson C.B. CD30 contains two binding sites with different specificities for members of the tumor necrosis factor receptor-associated factor family of signal transducing proteins. J. Biol. Chem. 1996;271:12852–12858. doi: 10.1074/jbc.271.22.12852. [DOI] [PubMed] [Google Scholar]
- 19.Lee S.Y., Park C.G., Choi Y. T cell receptor-dependent cell death of T cell hybridomas mediated by the CD30 cytoplasmic domain in association with tumor necrosis factor receptor-associated factors. J. Exp. Med. 1996;183:669–674. doi: 10.1084/jem.183.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jang I.K., Lee Z.H., Kim Y.J., Kim S.H., Kwon B.S. Human 4-1BB (CD137) signals are mediated by TRAF2 and activate nuclear factor-kappa B. Biochem. Biophys. Res. Commun. 1998;242:613–620. doi: 10.1006/bbrc.1997.8016. [DOI] [PubMed] [Google Scholar]
- 21.Saoulli K., Lee S.Y., Cannons J.L., Yeh W.C., Santana A., Goldstein M.D., Bangia N., DeBenedette M.A., Mak T.W., Choi Y., et al. CD28-independent, TRAF2-dependent costimulation of resting T cells by 4-1BB ligand. J. Exp. Med. 1998;187:1849–1862. doi: 10.1084/jem.187.11.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Darnay B.G., Haridas V., Ni J., Moore P.A., Aggarwal B.B. Characterization of the intracellular domain of receptor activator of NF-kappaB (RANK). Interaction with tumor necrosis factor receptor-associated factors and activation of NF-kappab and c-Jun N-terminal kinase. J. Biol. Chem. 1998;273:20551–20555. doi: 10.1074/jbc.273.32.20551. [DOI] [PubMed] [Google Scholar]
- 23.Galibert L., Tometsko M.E., Anderson D.M., Cosman D., Dougall W.C. The involvement of multiple tumor necrosis factor receptor (TNFR)-associated factors in the signaling mechanisms of receptor activator of NF-kappaB, a member of the TNFR superfamily. J. Biol. Chem. 1998;273:34120–34127. doi: 10.1074/jbc.273.51.34120. [DOI] [PubMed] [Google Scholar]
- 24.Wong B.R., Josien R., Lee S.Y., Vologodskaia M., Steinman R.M., Choi Y. The TRAF family of signal transducers mediates NF-kappaB activation by the TRANCE receptor. J. Biol. Chem. 1998;273:28355–28359. doi: 10.1074/jbc.273.43.28355. [DOI] [PubMed] [Google Scholar]
- 25.Wiley S.R., Cassiano L., Lofton T., Davis-Smith T., Winkles J.A., Lindner V., Liu H., Daniel T.O., Smith C.A., Fanslow W.C. A novel TNF receptor family member binds TWEAK and is implicated in angiogenesis. Immunity. 2001;15:837–846. doi: 10.1016/S1074-7613(01)00232-1. [DOI] [PubMed] [Google Scholar]
- 26.Xia X.Z., Treanor J., Senaldi G., Khare S.D., Boone T., Kelley M., Theill L.E., Colombero A., Solovyev I., Lee F., et al. TACI is a TRAF-interacting receptor for TALL-1, a tumor necrosis factor family member involved in B cell regulation. J. Exp. Med. 2000;192:137–143. doi: 10.1084/jem.192.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marsters S.A., Ayres T.M., Skubatch M., Gray C.L., Rothe M., Ashkenazi A. Herpesvirus entry mediator, a member of the tumor necrosis factor receptor (TNFR) family, interacts with members of the TNFR-associated factor family and activates the transcription factors NF-kappaB and AP-1. J. Biol. Chem. 1997;272:14029–14032. doi: 10.1074/jbc.272.22.14029. [DOI] [PubMed] [Google Scholar]
- 28.Ye X., Mehlen P., Rabizadeh S., VanArsdale T., Zhang H., Shin H., Wang J.J., Leo E., Zapata J., Hauser C.A., et al. TRAF family proteins interact with the common neurotrophin receptor and modulate apoptosis induction. J. Biol. Chem. 1999;274:30202–30208. doi: 10.1074/jbc.274.42.30202. [DOI] [PubMed] [Google Scholar]
- 29.Hatzoglou A., Roussel J., Bourgeade M.F., Rogier E., Madry C., Inoue J., Devergne O., Tsapis A. TNF receptor family member BCMA (B cell maturation) associates with TNF receptor-associated factor (TRAF) 1, TRAF2, and TRAF3 and activates NF-kappa B, elk-1, c-Jun N-terminal kinase, and p38 mitogen-activated protein kinase. J. Immunol. 2000;165:1322–1330. doi: 10.4049/jimmunol.165.3.1322. [DOI] [PubMed] [Google Scholar]
- 30.Esparza E.M., Arch R.H. Glucocorticoid-induced TNF receptor, a costimulatory receptor on naive and activated T cells, uses TNF receptor-associated factor 2 in a novel fashion as an inhibitor of NF-kappa B activation. J. Immunol. 2005;174:7875–7882. doi: 10.4049/jimmunol.174.12.7875. [DOI] [PubMed] [Google Scholar]
- 31.Kwon B., Yu K.Y., Ni J., Yu G.L., Jang I.K., Kim Y.J., Xing L., Liu D., Wang S.X., Kwon B.S. Identification of a novel activation-inducible protein of the tumor necrosis factor receptor superfamily and its ligand. J. Biol. Chem. 1999;274:6056–6061. doi: 10.1074/jbc.274.10.6056. [DOI] [PubMed] [Google Scholar]
- 32.Eby M.T., Jasmin A., Kumar A., Sharma K., Chaudhary P.M. TAJ, a novel member of the tumor necrosis factor receptor family, activates the c-Jun N-terminal kinase pathway and mediates caspase-independent cell death. J. Biol. Chem. 2000;275:15336–15342. doi: 10.1074/jbc.275.20.15336. [DOI] [PubMed] [Google Scholar]
- 33.Pereno R., Giron-Michel J., Gaggero A., Cazes E., Meazza R., Monetti M., Monaco E., Mishal Z., Jasmin C., Indiveri F., et al. IL-15/IL-15Ralpha intracellular trafficking in human melanoma cells and signal transduction through the IL-15Ralpha. Oncogene. 2000;19:5153–5162. doi: 10.1038/sj.onc.1203873. [DOI] [PubMed] [Google Scholar]
- 34.Yang C.H., Murti A., Pfeffer L.M. Interferon induces NF-kappa B-inducing kinase/tumor necrosis factor receptor-associated factor-dependent NF-kappa B activation to promote cell survival. J. Biol. Chem. 2005;280:31530–31536. doi: 10.1074/jbc.M503120200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaye K.M., Devergne O., Harada J.N., Izumi K.M., Yalamanchili R., Kieff E., Mosialos G. Tumor necrosis factor receptor associated factor 2 is a mediator of NF-kappa B activation by latent infection membrane protein 1, the Epstein-Barr virus transforming protein. Proc. Natl. Acad. Sci. USA. 1996;93:11085–11090. doi: 10.1073/pnas.93.20.11085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mosialos G., Birkenbach M., Yalamanchili R., VanArsdale T., Ware C., Kieff E. The Epstein-Barr virus transforming protein LMP1 engages signaling proteins for the tumor necrosis factor receptor family. Cell. 1995;80:389–399. doi: 10.1016/0092-8674(95)90489-1. [DOI] [PubMed] [Google Scholar]
- 37.Song H.Y., Rothe M., Goeddel D.V. The tumor necrosis factor-inducible zinc finger protein A20 interacts with TRAF1/TRAF2 and inhibits NF-kappaB activation. Proc. Natl. Acad. Sci. USA. 1996;93:6721–6725. doi: 10.1073/pnas.93.13.6721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang H., Zhang R., Luo Y., D’Alessio A., Pober J.S., Min W. AIP1/DAB2IP, a novel member of the Ras-GAP family, transduces TRAF2-induced ASK1-JNK activation. J. Biol. Chem. 2004;279:44955–44965. doi: 10.1074/jbc.M407617200. [DOI] [PubMed] [Google Scholar]
- 39.Choi J.W., Kim D.G., Park M.C., Um J.Y., Han J.M., Park S.G., Choi E.C., Kim S. AIMP2 promotes TNFalpha-dependent apoptosis via ubiquitin-mediated degradation of TRAF2. J. Cell Sci. 2009;122:2710–2715. doi: 10.1242/jcs.049767. [DOI] [PubMed] [Google Scholar]
- 40.Hupalowska A., Pyrzynska B., Miaczynska M. APPL1 regulates basal NF-κB activity by stabilizing NIK. J. Cell Sci. 2012;125:4090–4102. doi: 10.1242/jcs.105171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chang E.J., Ha J., Kang S.S., Lee Z.H., Kim H.H. AWP1 binds to tumor necrosis factor receptor-associated factor 2 (TRAF2) and is involved in TRAF2-mediated nuclear factor-kappaB signaling. Int J. Biochem. Cell Biol. 2011;43:1612–1620. doi: 10.1016/j.biocel.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 42.Yoneda T., Imaizumi K., Maeda M., Yui D., Manabe T., Katayama T., Sato N., Gomi F., Morihara T., Mori Y., et al. Regulatory mechanisms of TRAF2-mediated signal transduction by Bcl10, a MALT lymphoma-associated protein. J. Biol. Chem. 2000;275:11114–11120. doi: 10.1074/jbc.275.15.11114. [DOI] [PubMed] [Google Scholar]
- 43.Liu H., Ma Y., He H.W., Zhao W.L., Shao R.G. SPHK1 (sphingosine kinase 1) induces epithelial-mesenchymal transition by promoting the autophagy-linked lysosomal degradation of CDH1/E-cadherin in hepatoma cells. Autophagy. 2017;13:900–913. doi: 10.1080/15548627.2017.1291479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lamkanfi M., D’Hondt K., Vande Walle L., van Gurp M., Denecker G., Demeulemeester J., Kalai M., Declercq W., Saelens X., Vandenabeele P. A novel caspase-2 complex containing TRAF2 and RIP1. J. Biol. Chem. 2005;280:6923–6932. doi: 10.1074/jbc.M411180200. [DOI] [PubMed] [Google Scholar]
- 45.Yoneda T., Imaizumi K., Oono K., Yui D., Gomi F., Katayama T., Tohyama M. Activation of caspase-12, an endoplastic reticulum (ER) resident caspase, through tumor necrosis factor receptor-associated factor 2-dependent mechanism in response to the ER stress. J. Biol. Chem. 2001;276:13935–13940. doi: 10.1074/jbc.M010677200. [DOI] [PubMed] [Google Scholar]
- 46.Yan R., Zhu H., Huang P., Yang M., Shen M., Pan Y., Zhang C., Zhou X., Li H., Ke X., et al. Liquidambaric acid inhibits Wnt/β-catenin signaling and colon cancer via targeting TNF receptor-associated factor 2. Cell Rep. 2022;38:110319. doi: 10.1016/j.celrep.2022.110319. [DOI] [PubMed] [Google Scholar]
- 47.Feng X., Gaeta M.L., Madge L.A., Yang J.H., Bradley J.R., Pober J.S. Caveolin-1 associates with TRAF2 to form a complex that is recruited to tumor necrosis factor receptors. J. Biol. Chem. 2001;276:8341–8349. doi: 10.1074/jbc.M007116200. [DOI] [PubMed] [Google Scholar]
- 48.Jang K.W., Lee K.H., Kim S.H., Jin T., Choi E.Y., Jeon H.J., Kim E., Han Y.S., Chung J.H. Ubiquitin ligase CHIP induces TRAF2 proteasomal degradation and NF-κB inactivation to regulate breast cancer cell invasion. J. Cell Biochem. 2011;112:3612–3620. doi: 10.1002/jcb.23292. [DOI] [PubMed] [Google Scholar]
- 49.Kovalenko A., Chable-Bessia C., Cantarella G., Israël A., Wallach D., Courtois G. The tumour suppressor CYLD negatively regulates NF-kappaB signalling by deubiquitination. Nature. 2003;424:801–805. doi: 10.1038/nature01802. [DOI] [PubMed] [Google Scholar]
- 50.Yang C.Y., Chiu L.L., Tan T.H. TRAF2-mediated Lys63-linked ubiquitination of DUSP14/MKP6 is essential for its phosphatase activity. Cell Signal. 2016;28:145–151. doi: 10.1016/j.cellsig.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 51.Zhang P., Zhang Z., Fu Y., Zhang Y., Washburn M.P., Florens L., Wu M., Huang C., Hou Z., Mohan M. K63-linked ubiquitination of DYRK1A by TRAF2 alleviates Sprouty 2-mediated degradation of EGFR. Cell Death Dis. 2021;12:608. doi: 10.1038/s41419-021-03887-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blakely C.M., Pazarentzos E., Olivas V., Asthana S., Yan J.J., Tan I., Hrustanovic G., Chan E., Lin L., Neel D.S., et al. NF-κB-activating complex engaged in response to EGFR oncogene inhibition drives tumor cell survival and residual disease in lung cancer. Cell Rep. 2015;11:98–110. doi: 10.1016/j.celrep.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choi J.M., Devkota S., Sung Y.H., Lee H.W. EI24 regulates epithelial-to-mesenchymal transition and tumor progression by suppressing TRAF2-mediated NF-κB activity. Oncotarget. 2013;4:2383–2396. doi: 10.18632/oncotarget.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim W.J., Back S.H., Kim V., Ryu I., Jang S.K. Sequestration of TRAF2 into stress granules interrupts tumor necrosis factor signaling under stress conditions. Mol. Cell Biol. 2005;25:2450–2462. doi: 10.1128/MCB.25.6.2450-2462.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ohtsu N., Nakatani Y., Yamashita D., Ohue S., Ohnishi T., Kondo T. Eva1 Maintains the Stem-like Character of Glioblastoma-Initiating Cells by Activating the Noncanonical NF-κB Signaling Pathway. Cancer Res. 2016;76:171–181. doi: 10.1158/0008-5472.CAN-15-0884. [DOI] [PubMed] [Google Scholar]
- 56.da Silva S.D., Xu B., Maschietto M., Marchi F.A., Alkailani M.I., Bijian K., Xiao D., Alaoui-Jamali M.A. TRAF2 Cooperates with Focal Adhesion Signaling to Regulate Cancer Cell Susceptibility to Anoikis. Mol. Cancer Ther. 2019;18:139–146. doi: 10.1158/1535-7163.MCT-17-1261. [DOI] [PubMed] [Google Scholar]
- 57.Leonardi A., Ellinger-Ziegelbauer H., Franzoso G., Brown K., Siebenlist U. Physical and functional interaction of filamin (actin-binding protein-280) and tumor necrosis factor receptor-associated factor 2. J. Biol. Chem. 2000;275:271–278. doi: 10.1074/jbc.275.1.271. [DOI] [PubMed] [Google Scholar]
- 58.Shi C.S., Leonardi A., Kyriakis J., Siebenlist U., Kehrl J.H. TNF-mediated activation of the stress-activated protein kinase pathway: TNF receptor-associated factor 2 recruits and activates germinal center kinase related. J. Immunol. 1999;163:3279–3285. [PubMed] [Google Scholar]
- 59.Lee S., Lee E.K., Kang D.H., Lee J., Hong S.H., Jeong W., Kang S.W. Glutathione peroxidase-1 regulates ASK1-dependent apoptosis via interaction with TRAF2 in RIPK3-negative cancer cells. Exp. Mol. Med. 2021;53:1080–1091. doi: 10.1038/s12276-021-00642-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sangaré L.O., Yang N., Konstantinou E.K., Lu D., Mukhopadhyay D., Young L.H., Saeij J.P.J. Toxoplasma GRA15 Activates the NF-κB Pathway through Interactions with TNF Receptor-Associated Factors. mBio. 2019;10:e00808-19. doi: 10.1128/mBio.00808-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu Y., Fan Y., Xue B., Luo L., Shen J., Zhang S., Jiang Y., Yin Z. Human glutathione S-transferase P1-1 interacts with TRAF2 and regulates TRAF2-ASK1 signals. Oncogene. 2006;25:5787–5800. doi: 10.1038/sj.onc.1209576. [DOI] [PubMed] [Google Scholar]
- 62.Chuang H.C., Sheu W.H., Lin Y.T., Tsai C.Y., Yang C.Y., Cheng Y.J., Huang P.Y., Li J.P., Chiu L.L., Wang X., et al. HGK/MAP4K4 deficiency induces TRAF2 stabilization and Th17 differentiation leading to insulin resistance. Nat. Commun. 2014;5:4602. doi: 10.1038/ncomms5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lambert B., Vandeputte J., Remacle S., Bergiers I., Simonis N., Twizere J.C., Vidal M., Rezsohazy R. Protein interactions of the transcription factor Hoxa1. BMC Dev. Biol. 2012;12:29. doi: 10.1186/1471-213X-12-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dai S., Jiang L., Wang G., Zhou X., Wei X., Cheng H., Wu Z., Wei D. HSP70 interacts with TRAF2 and differentially regulates TNFalpha signalling in human colon cancer cells. J. Cell Mol. Med. 2010;14:710–725. doi: 10.1111/j.1582-4934.2009.00716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Devin A., Lin Y., Yamaoka S., Li Z., Karin M., Liu Z. The alpha and beta subunits of IkappaB kinase (IKK) mediate TRAF2-dependent IKK recruitment to tumor necrosis factor (TNF) receptor 1 in response to TNF. Mol. Cell Biol. 2001;21:3986–3994. doi: 10.1128/MCB.21.12.3986-3994.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou A.Y., Shen R.R., Kim E., Lock Y.J., Xu M., Chen Z.J., Hahn W.C. IKKε-mediated tumorigenesis requires K63-linked polyubiquitination by a cIAP1/cIAP2/TRAF2 E3 ubiquitin ligase complex. Cell Rep. 2013;3:724–733. doi: 10.1016/j.celrep.2013.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Urano F., Wang X., Bertolotti A., Zhang Y., Chung P., Harding H.P., Ron D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 68.Rothe M., Xiong J., Shu H.B., Williamson K., Goddard A., Goeddel D.V. I-TRAF is a novel TRAF-interacting protein that regulates TRAF-mediated signal transduction. Proc. Natl. Acad. Sci. USA. 1996;93:8241–8246. doi: 10.1073/pnas.93.16.8241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Oukka M., Kim S.T., Lugo G., Sun J., Wu L.C., Glimcher L.H. A mammalian homolog of Drosophila schnurri, KRC, regulates TNF receptor-driven responses and interacts with TRAF2. Mol. Cell. 2002;9:121–131. doi: 10.1016/S1097-2765(01)00434-8. [DOI] [PubMed] [Google Scholar]
- 70.Parisien J.P., Lenoir J.J., Mandhana R., Rodriguez K.R., Qian K., Bruns A.M., Horvath C.M. RNA sensor LGP2 inhibits TRAF ubiquitin ligase to negatively regulate innate immune signaling. EMBO Rep. 2018;19:e45176. doi: 10.15252/embr.201745176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu G., Xu Y., Schultz R.D., Chen H., Xie J., Deng M., Liu X., Gui X., John S., Lu Z., et al. LILRB3 supports acute myeloid leukemia development and regulates T-cell antitumor immune responses through the TRAF2-cFLIP-NF-κB signaling axis. Nat. Cancer. 2021;2:1170–1184. doi: 10.1038/s43018-021-00262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jia H., Yang Y., Li M., Chu Y., Song H., Zhang J., Zhang D., Zhang Q., Xu Y., Wang J., et al. Snail enhances arginine synthesis by inhibiting ubiquitination-mediated degradation of ASS1. EMBO Rep. 2021;22:e51780. doi: 10.15252/embr.202051780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xu L.G., Wang Y.Y., Han K.J., Li L.Y., Zhai Z., Shu H.B. VISA is an adapter protein required for virus-triggered IFN-beta signaling. Mol. Cell. 2005;19:727–740. doi: 10.1016/j.molcel.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 74.Fang R., Jiang Q., Zhou X., Wang C., Guan Y., Tao J., Xi J., Feng J.M., Jiang Z. MAVS activates TBK1 and IKKε through TRAFs in NEMO dependent and independent manner. PLoS Pathog. 2017;13:e1006720. doi: 10.1371/journal.ppat.1006720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baud V., Liu Z.G., Bennett B., Suzuki N., Xia Y., Karin M. Signaling by proinflammatory cytokines: Oligomerization of TRAF2 and TRAF6 is sufficient for JNK and IKK activation and target gene induction via an amino-terminal effector domain. Genes Dev. 1999;13:1297–1308. doi: 10.1101/gad.13.10.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu J., Yan J., Jiang S., Wen J., Chen L., Zhao Y., Lin A. Site-specific ubiquitination is required for relieving the transcription factor Miz1-mediated suppression on TNF-α-induced JNK activation and inflammation. Proc. Natl. Acad. Sci. USA. 2012;109:191–196. doi: 10.1073/pnas.1105176108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Korchnak A.C., Zhan Y., Aguilar M.T., Chadee D.N. Cytokine-induced activation of mixed lineage kinase 3 requires TRAF2 and TRAF6. Cell. Signal. 2009;21:1620–1625. doi: 10.1016/j.cellsig.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sondarva G., Kundu C.N., Mehrotra S., Mishra R., Rangasamy V., Sathyanarayana P., Ray R.S., Rana B., Rana A. TRAF2-MLK3 interaction is essential for TNF-alpha-induced MLK3 activation. Cell Res. 2010;20:89–98. doi: 10.1038/cr.2009.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Roh K.H., Choi E.J. TRAF2 functions as an activator switch in the reactive oxygen species-induced stimulation of MST1. Free Radic. Biol. Med. 2016;91:105–113. doi: 10.1016/j.freeradbiomed.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 80.Zapata J.M., Pawlowski K., Haas E., Ware C.F., Godzik A., Reed J.C. A diverse family of proteins containing tumor necrosis factor receptor-associated factor domains. J. Biol. Chem. 2001;276:24242–24252. doi: 10.1074/jbc.M100354200. [DOI] [PubMed] [Google Scholar]
- 81.Khan K.A., Abbas W., Varin A., Kumar A., Di Martino V., Dichamp I., Herbein G. HIV-1 Nef interacts with HCV Core, recruits TRAF2, TRAF5 and TRAF6, and stimulates HIV-1 replication in macrophages. J. Innate Immun. 2013;5:639–656. doi: 10.1159/000350517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mangino G., Percario Z.A., Fiorucci G., Vaccari G., Acconcia F., Chiarabelli C., Leone S., Noto A., Horenkamp F.A., Manrique S., et al. HIV-1 Nef induces proinflammatory state in macrophages through its acidic cluster domain: Involvement of TNF alpha receptor associated factor 2. PLoS ONE. 2011;6:e22982. doi: 10.1371/journal.pone.0022982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lieberson R., Mowen K.A., McBride K.D., Leautaud V., Zhang X., Suh W.K., Wu L., Glimcher L.H. Tumor necrosis factor receptor-associated factor (TRAF)2 represses the T helper cell type 2 response through interaction with NFAT-interacting protein (NIP45) J. Exp. Med. 2001;194:89–98. doi: 10.1084/jem.194.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bagchi P., Bhowmick R., Nandi S., Kant Nayak M., Chawla-Sarkar M. Rotavirus NSP1 inhibits interferon induced non-canonical NFκB activation by interacting with TNF receptor associated factor 2. Virology. 2013;444:41–44. doi: 10.1016/j.virol.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 85.Hu M., Luo Q., Alitongbieke G., Chong S., Xu C., Xie L., Chen X., Zhang D., Zhou Y., Wang Z., et al. Celastrol-Induced Nur77 Interaction with TRAF2 Alleviates Inflammation by Promoting Mitochondrial Ubiquitination and Autophagy. Mol. Cell. 2017;66:141–153.e146. doi: 10.1016/j.molcel.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Henn I.H., Bouman L., Schlehe J.S., Schlierf A., Schramm J.E., Wegener E., Nakaso K., Culmsee C., Berninger B., Krappmann D., et al. Parkin mediates neuroprotection through activation of IkappaB kinase/nuclear factor-kappaB signaling. J. Neurosci. 2007;27:1868–1878. doi: 10.1523/JNEUROSCI.5537-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sorokin A.V., Nair B.C., Wei Y., Aziz K.E., Evdokimova V., Hung M.C., Chen J. Aberrant Expression of proPTPRN2 in Cancer Cells Confers Resistance to Apoptosis. Cancer Res. 2015;75:1846–1858. doi: 10.1158/0008-5472.CAN-14-2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wixted J.H., Rothstein J.L., Eisenlohr L.C. Identification of functionally distinct TRAF proinflammatory and phosphatidylinositol 3-kinase/mitogen-activated protein kinase/extracellular signal-regulated kinase kinase (PI3K/MEK) transforming activities emanating from RET/PTC fusion oncoprotein. J. Biol. Chem. 2012;287:3691–3703. doi: 10.1074/jbc.M111.322677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Takeuchi M., Rothe M., Goeddel D.V. Anatomy of TRAF2. Distinct domains for nuclear factor-kappaB activation and association with tumor necrosis factor signaling proteins. J. Biol. Chem. 1996;271:19935–19942. doi: 10.1074/jbc.271.33.19935. [DOI] [PubMed] [Google Scholar]
- 90.Thome M., Hofmann K., Burns K., Martinon F., Bodmer J.L., Mattmann C., Tschopp J. Identification of CARDIAK, a RIP-like kinase that associates with caspase-1. Curr. Biol. 1998;8:885–888. doi: 10.1016/S0960-9822(07)00352-1. [DOI] [PubMed] [Google Scholar]
- 91.Wang Q., Jiang M., Wu J., Ma Y., Li T., Chen Q., Zhang X., Xiang L. Stress-induced RNASET2 overexpression mediates melanocyte apoptosis via the TRAF2 pathway in vitro. Cell Death Dis. 2014;5:e1022. doi: 10.1038/cddis.2013.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Peng C., Zhu F., Wen W., Yao K., Li S., Zykova T., Liu K., Li X., Ma W.Y., Bode A.M., et al. Tumor necrosis factor receptor-associated factor family protein 2 is a key mediator of the epidermal growth factor-induced ribosomal S6 kinase 2/cAMP-responsive element-binding protein/Fos protein signaling pathway. J. Biol. Chem. 2012;287:25881–25892. doi: 10.1074/jbc.M112.359521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Busca A., Konarski Y., Gajanayaka N., O’Hara S., Angel J., Kozlowski M., Kumar A. cIAP1/2-TRAF2-SHP-1-Src-MyD88 Complex Regulates Lipopolysaccharide-Induced IL-27 Production through NF-κB Activation in Human Macrophages. J. Immunol. 2018;200:1593–1606. doi: 10.4049/jimmunol.1700199. [DOI] [PubMed] [Google Scholar]
- 94.Fortin Ensign S.P., Mathews I.T., Eschbacher J.M., Loftus J.C., Symons M.H., Tran N.L. The Src homology 3 domain-containing guanine nucleotide exchange factor is overexpressed in high-grade gliomas and promotes tumor necrosis factor-like weak inducer of apoptosis-fibroblast growth factor-inducible 14-induced cell migration and invasion via tumor necrosis factor receptor-associated factor 2. J. Biol. Chem. 2013;288:21887–21897. doi: 10.1074/jbc.M113.468686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liang Y. SHARPIN negatively associates with TRAF2-mediated NFκB activation. PLoS ONE. 2011;6:e21696. doi: 10.1371/journal.pone.0021696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Habelhah H., Frew I.J., Laine A., Janes P.W., Relaix F., Sassoon D., Bowtell D.D., Ronai Z. Stress-induced decrease in TRAF2 stability is mediated by Siah2. Embo. J. 2002;21:5756–5765. doi: 10.1093/emboj/cdf576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shimada K., Ikeda K., Ito K. Traf2 interacts with Smad4 and regulates BMP signaling pathway in MC3T3-E1 osteoblasts. Biochem. Biophys. Res. Commun. 2009;390:775–779. doi: 10.1016/j.bbrc.2009.10.048. [DOI] [PubMed] [Google Scholar]
- 98.Carpentier I., Coornaert B., Beyaert R. Smurf2 is a TRAF2 binding protein that triggers TNF-R2 ubiquitination and TNF-R2-induced JNK activation. Biochem. Biophys. Res. Commun. 2008;374:752–757. doi: 10.1016/j.bbrc.2008.07.103. [DOI] [PubMed] [Google Scholar]
- 99.Wu W., Wang J., Xiao C., Su Z., Su H., Zhong W., Mao J., Liu X., Zhu Y.Z. SMYD2-mediated TRAF2 methylation promotes the NF-κB signaling pathways in inflammatory diseases. Clin. Transl. Med. 2021;11:e591. doi: 10.1002/ctm2.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xia P., Wang L., Moretti P.A., Albanese N., Chai F., Pitson S.M., D’Andrea R.J., Gamble J.R., Vadas M.A. Sphingosine kinase interacts with TRAF2 and dissects tumor necrosis factor-alpha signaling. J. Biol. Chem. 2002;277:7996–8003. doi: 10.1074/jbc.M111423200. [DOI] [PubMed] [Google Scholar]
- 101.Kanamori M., Suzuki H., Saito R., Muramatsu M., Hayashizaki Y. T2BP, a novel TRAF2 binding protein, can activate NF-kappaB and AP-1 without TNF stimulation. Biochem. Biophys. Res. Commun. 2002;290:1108–1113. doi: 10.1006/bbrc.2001.6315. [DOI] [PubMed] [Google Scholar]
- 102.van Vliet C., Bukczynska P.E., Puryer M.A., Sadek C.M., Shields B.J., Tremblay M.L., Tiganis T. Selective regulation of tumor necrosis factor-induced Erk signaling by Src family kinases and the T cell protein tyrosine phosphatase. Nat. Immunol. 2005;6:253–260. doi: 10.1038/ni1169. [DOI] [PubMed] [Google Scholar]
- 103.Eliopoulos A.G., Davies C., Blake S.S., Murray P., Najafipour S., Tsichlis P.N., Young L.S. The oncogenic protein kinase Tpl-2/Cot contributes to Epstein-Barr virus-encoded latent infection membrane protein 1-induced NF-kappaB signaling downstream of TRAF2. J. Virol. 2002;76:4567–4579. doi: 10.1128/JVI.76.9.4567-4579.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hong S., Lim S., Li A.G., Lee C., Lee Y.S., Lee E.K., Park S.H., Wang X.J., Kim S.J. Smad7 binds to the adaptors TAB2 and TAB3 to block recruitment of the kinase TAK1 to the adaptor TRAF2. Nat. Immunol. 2007;8:504–513. doi: 10.1038/ni1451. [DOI] [PubMed] [Google Scholar]
- 105.Fu C.A., Shen M., Huang B.C., Lasaga J., Payan D.G., Luo Y. TNIK, a novel member of the germinal center kinase family that activates the c-Jun N-terminal kinase pathway and regulates the cytoskeleton. J. Biol. Chem. 1999;274:30729–30737. doi: 10.1074/jbc.274.43.30729. [DOI] [PubMed] [Google Scholar]
- 106.Hsu H., Shu H.B., Pan M.G., Goeddel D.V. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell. 1996;84:299–308. doi: 10.1016/S0092-8674(00)80984-8. [DOI] [PubMed] [Google Scholar]
- 107.Lu C.H., Yeh D.W., Lai C.Y., Liu Y.L., Huang L.R., Lee A.Y., Jin S.C., Chuang T.H. USP17 mediates macrophage-promoted inflammation and stemness in lung cancer cells by regulating TRAF2/TRAF3 complex formation. Oncogene. 2018;37:6327–6340. doi: 10.1038/s41388-018-0411-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang X., Wen Z., Sun L., Wang J., Song M., Wang E., Mi X. TRAF2 regulates the cytoplasmic/nuclear distribution of TRAF4 and its biological function in breast cancer cells. Biochem. Biophys. Res. Commun. 2013;436:344–348. doi: 10.1016/j.bbrc.2013.05.107. [DOI] [PubMed] [Google Scholar]
- 109.Sasai M., Tatematsu M., Oshiumi H., Funami K., Matsumoto M., Hatakeyama S., Seya T. Direct binding of TRAF2 and TRAF6 to TICAM-1/TRIF adaptor participates in activation of the Toll-like receptor 3/4 pathway. Mol. Immunol. 2010;47:1283–1291. doi: 10.1016/j.molimm.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 110.Xiao N., Li H., Luo J., Wang R., Chen H., Chen J., Wang P. Ubiquitin-specific protease 4 (USP4) targets TRAF2 and TRAF6 for deubiquitination and inhibits TNFα-induced cancer cell migration. Biochem. J. 2012;441:979–986. doi: 10.1042/BJ20111358. [DOI] [PubMed] [Google Scholar]
- 111.Huang Y., Chen L., Zhou Y., Liu H., Yang J., Liu Z., Wang C. UXT-V1 protects cells against TNF-induced apoptosis through modulating complex II formation. Mol. Biol. Cell. 2011;22:1389–1397. doi: 10.1091/mbc.e10-10-0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.LaMonica R., Kocer S.S., Nazarova J., Dowling W., Geimonen E., Shaw R.D., Mackow E.R. VP4 differentially regulates TRAF2 signaling, disengaging JNK activation while directing NF-kappa B to effect rotavirus-specific cellular responses. J. Biol. Chem. 2001;276:19889–19896. doi: 10.1074/jbc.M100499200. [DOI] [PubMed] [Google Scholar]
- 113.Prinz E., Aviram S., Aronheim A. WDR62 mediates TNFα-dependent JNK activation via TRAF2-MLK3 axis. Mol. Biol. Cell. 2018;29:2470–2480. doi: 10.1091/mbc.E17-08-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gonzalvez F., Lawrence D., Yang B., Yee S., Pitti R., Marsters S., Pham V.C., Stephan J.P., Lill J., Ashkenazi A. TRAF2 Sets a threshold for extrinsic apoptosis by tagging caspase-8 with a ubiquitin shutoff timer. Mol. Cell. 2012;48:888–899. doi: 10.1016/j.molcel.2012.09.031. [DOI] [PubMed] [Google Scholar]
- 115.Xu L., Zhang Y., Qu X., Che X., Guo T., Li C., Ma R., Fan Y., Ma Y., Hou K., et al. DR5-Cbl-b/c-Cbl-TRAF2 complex inhibits TRAIL-induced apoptosis by promoting TRAF2-mediated polyubiquitination of caspase-8 in gastric cancer cells. Mol. Oncol. 2017;11:1733–1751. doi: 10.1002/1878-0261.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jin J., Xiao Y., Hu H., Zou Q., Li Y., Gao Y., Ge W., Cheng X., Sun S.C. Proinflammatory TLR signalling is regulated by a TRAF2-dependent proteolysis mechanism in macrophages. Nat. Commun. 2015;6:5930. doi: 10.1038/ncomms6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shen Y., Liu W.W., Zhang X., Shi J.G., Jiang S., Zheng L., Qin Y., Liu B., Shi J.H. TRAF3 promotes ROS production and pyroptosis by targeting ULK1 ubiquitination in macrophages. Faseb. J. 2020;34:7144–7159. doi: 10.1096/fj.201903073R. [DOI] [PubMed] [Google Scholar]
- 118.Yang K.C., Ma X., Liu H., Murphy J., Barger P.M., Mann D.L., Diwan A. Tumor necrosis factor receptor-associated factor 2 mediates mitochondrial autophagy. Circ. Heart Fail. 2015;8:175–187. doi: 10.1161/CIRCHEARTFAILURE.114.001635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zachari M., Gudmundsson S.R., Li Z., Manifava M., Cugliandolo F., Shah R., Smith M., Stronge J., Karanasios E., Piunti C., et al. Selective Autophagy of Mitochondria on a Ubiquitin-Endoplasmic-Reticulum Platform. Dev. Cell. 2019;50:627–643.e625. doi: 10.1016/j.devcel.2019.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sun S.C. The non-canonical NF-κB pathway in immunity and inflammation. Nat. Rev. Immunol. 2017;17:545–558. doi: 10.1038/nri.2017.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang Q., Lenardo M.J., Baltimore D. 30 Years of NF-κB: A Blossoming of Relevance to Human Pathobiology. Cell. 2017;168:37–57. doi: 10.1016/j.cell.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Brummelkamp T.R., Nijman S.M., Dirac A.M., Bernards R. Loss of the cylindromatosis tumour suppressor inhibits apoptosis by activating NF-kappaB. Nature. 2003;424:797–801. doi: 10.1038/nature01811. [DOI] [PubMed] [Google Scholar]
- 123.Tesio M., Tang Y., Müdder K., Saini M., von Paleske L., Macintyre E., Pasparakis M., Waisman A., Trumpp A. Hematopoietic stem cell quiescence and function are controlled by the CYLD-TRAF2-p38MAPK pathway. J. Exp. Med. 2015;212:525–538. doi: 10.1084/jem.20141438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Trompouki E., Hatzivassiliou E., Tsichritzis T., Farmer H., Ashworth A., Mosialos G. CYLD is a deubiquitinating enzyme that negatively regulates NF-kappaB activation by TNFR family members. Nature. 2003;424:793–796. doi: 10.1038/nature01803. [DOI] [PubMed] [Google Scholar]
- 125.Lork M., Verhelst K., Beyaert R. CYLD, A20 and OTULIN deubiquitinases in NF-κB signaling and cell death: So similar, yet so different. Cell Death Differ. 2017;24:1172–1183. doi: 10.1038/cdd.2017.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Borghi A., Haegman M., Fischer R., Carpentier I., Bertrand M.J.M., Libert C., Afonina I.S., Beyaert R. The E3 ubiquitin ligases HOIP and cIAP1 are recruited to the TNFR2 signaling complex and mediate TNFR2-induced canonical NF-κB signaling. Biochem. Pharmacol. 2018;153:292–298. doi: 10.1016/j.bcp.2018.01.039. [DOI] [PubMed] [Google Scholar]
- 127.Lafont E., Kantari-Mimoun C., Draber P., De Miguel D., Hartwig T., Reichert M., Kupka S., Shimizu Y., Taraborrelli L., Spit M., et al. The linear ubiquitin chain assembly complex regulates TRAIL-induced gene activation and cell death. Embo. J. 2017;36:1147–1166. doi: 10.15252/embj.201695699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tokunaga F., Nakagawa T., Nakahara M., Saeki Y., Taniguchi M., Sakata S., Tanaka K., Nakano H., Iwai K. SHARPIN is a component of the NF-κB-activating linear ubiquitin chain assembly complex. Nature. 2011;471:633–636. doi: 10.1038/nature09815. [DOI] [PubMed] [Google Scholar]
- 129.Varfolomeev E., Goncharov T., Maecker H., Zobel K., Kömüves L.G., Deshayes K., Vucic D. Cellular inhibitors of apoptosis are global regulators of NF-κB and MAPK activation by members of the TNF family of receptors. Sci. Signal. 2012;5:ra22. doi: 10.1126/scisignal.2001878. [DOI] [PubMed] [Google Scholar]
- 130.Xie P., Hostager B.S., Munroe M.E., Moore C.R., Bishop G.A. Cooperation between TNF receptor-associated factors 1 and 2 in CD40 signaling. J. Immunol. 2006;176:5388–5400. doi: 10.4049/jimmunol.176.9.5388. [DOI] [PubMed] [Google Scholar]
- 131.Rowland S.L., Tremblay M.M., Ellison J.M., Stunz L.L., Bishop G.A., Hostager B.S. A novel mechanism for TNFR-associated factor 6-dependent CD40 signaling. J. Immunol. 2007;179:4645–4653. doi: 10.4049/jimmunol.179.7.4645. [DOI] [PubMed] [Google Scholar]
- 132.Vallabhapurapu S., Matsuzawa A., Zhang W., Tseng P.H., Keats J.J., Wang H., Vignali D.A., Bergsagel P.L., Karin M. Nonredundant and complementary functions of TRAF2 and TRAF3 in a ubiquitination cascade that activates NIK-dependent alternative NF-kappaB signaling. Nat. Immunol. 2008;9:1364–1370. doi: 10.1038/ni.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zarnegar B.J., Wang Y., Mahoney D.J., Dempsey P.W., Cheung H.H., He J., Shiba T., Yang X., Yeh W.C., Mak T.W., et al. Noncanonical NF-kappaB activation requires coordinated assembly of a regulatory complex of the adaptors cIAP1, cIAP2, TRAF2 and TRAF3 and the kinase NIK. Nat. Immunol. 2008;9:1371–1378. doi: 10.1038/ni.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Fotin-Mleczek M., Henkler F., Samel D., Reichwein M., Hausser A., Parmryd I., Scheurich P., Schmid J.A., Wajant H. Apoptotic crosstalk of TNF receptors: TNF-R2-induces depletion of TRAF2 and IAP proteins and accelerates TNF-R1-dependent activation of caspase-8. J. Cell Sci. 2002;115:2757–2770. doi: 10.1242/jcs.115.13.2757. [DOI] [PubMed] [Google Scholar]
- 135.Vince J.E., Chau D., Callus B., Wong W.W., Hawkins C.J., Schneider P., McKinlay M., Benetatos C.A., Condon S.M., Chunduru S.K., et al. TWEAK-FN14 signaling induces lysosomal degradation of a cIAP1-TRAF2 complex to sensitize tumor cells to TNFalpha. J. Cell Biol. 2008;182:171–184. doi: 10.1083/jcb.200801010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ablasser A., Hur S. Regulation of cGAS- and RLR-mediated immunity to nucleic acids. Nat. Immunol. 2020;21:17–29. doi: 10.1038/s41590-019-0556-1. [DOI] [PubMed] [Google Scholar]
- 137.Cadena C., Ahmad S., Xavier A., Willemsen J., Park S., Park J.W., Oh S.W., Fujita T., Hou F., Binder M., et al. Ubiquitin-Dependent and -Independent Roles of E3 Ligase RIPLET in Innate Immunity. Cell. 2019;177:1187–1200.e1116. doi: 10.1016/j.cell.2019.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jiang X., Kinch L.N., Brautigam C.A., Chen X., Du F., Grishin N.V., Chen Z.J. Ubiquitin-induced oligomerization of the RNA sensors RIG-I and MDA5 activates antiviral innate immune response. Immunity. 2012;36:959–973. doi: 10.1016/j.immuni.2012.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Peisley A., Wu B., Yao H., Walz T., Hur S. RIG-I forms signaling-competent filaments in an ATP-dependent, ubiquitin-independent manner. Mol. Cell. 2013;51:573–583. doi: 10.1016/j.molcel.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 140.Wu B., Peisley A., Tetrault D., Li Z., Egelman E.H., Magor K.E., Walz T., Penczek P.A., Hur S. Molecular imprinting as a signal-activation mechanism of the viral RNA sensor RIG-I. Mol. Cell. 2014;55:511–523. doi: 10.1016/j.molcel.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Liu S., Chen J., Cai X., Wu J., Chen X., Wu Y.T., Sun L., Chen Z.J. MAVS recruits multiple ubiquitin E3 ligases to activate antiviral signaling cascades. Elife. 2013;2:e00785. doi: 10.7554/eLife.00785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Liu S., Cai X., Wu J., Cong Q., Chen X., Li T., Du F., Ren J., Wu Y.T., Grishin N.V., et al. Phosphorylation of innate immune adaptor proteins MAVS, STING, and TRIF induces IRF3 activation. Science. 2015;347:aaa2630. doi: 10.1126/science.aaa2630. [DOI] [PubMed] [Google Scholar]
- 143.Shu H.B., Takeuchi M., Goeddel D.V. The tumor necrosis factor receptor 2 signal transducers TRAF2 and c-IAP1 are components of the tumor necrosis factor receptor 1 signaling complex. Proc. Natl. Acad. Sci. USA. 1996;93:13973–13978. doi: 10.1073/pnas.93.24.13973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang C.Y., Mayo M.W., Korneluk R.G., Goeddel D.V., Baldwin A.S., Jr. NF-kappaB antiapoptosis: Induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- 145.Weiss T., Grell M., Siemienski K., Mühlenbeck F., Dürkop H., Pfizenmaier K., Scheurich P., Wajant H. TNFR80-dependent enhancement of TNFR60-induced cell death is mediated by TNFR-associated factor 2 and is specific for TNFR60. J. Immunol. 1998;161:3136–3142. [PubMed] [Google Scholar]
- 146.Yeh W.C., Shahinian A., Speiser D., Kraunus J., Billia F., Wakeham A., de la Pompa J.L., Ferrick D., Hum B., Iscove N., et al. Early lethality, functional NF-kappaB activation, and increased sensitivity to TNF-induced cell death in TRAF2-deficient mice. Immunity. 1997;7:715–725. doi: 10.1016/S1074-7613(00)80391-X. [DOI] [PubMed] [Google Scholar]
- 147.Chan F.K., Lenardo M.J. A crucial role for p80 TNF-R2 in amplifying p60 TNF-R1 apoptosis signals in T lymphocytes. Eur. J. Immunol. 2000;30:652–660. doi: 10.1002/1521-4141(200002)30:2<652::AID-IMMU652>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 148.Duckett C.S., Thompson C.B. CD30-dependent degradation of TRAF2: Implications for negative regulation of TRAF signaling and the control of cell survival. Genes Dev. 1997;11:2810–2821. doi: 10.1101/gad.11.21.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Li X., Yang Y., Ashwell J.D. TNF-RII and c-IAP1 mediate ubiquitination and degradation of TRAF2. Nature. 2002;416:345–347. doi: 10.1038/416345a. [DOI] [PubMed] [Google Scholar]
- 150.Weiss T., Grell M., Hessabi B., Bourteele S., Müller G., Scheurich P., Wajant H. Enhancement of TNF receptor p60-mediated cytotoxicity by TNF receptor p80: Requirement of the TNF receptor-associated factor-2 binding site. J. Immunol. 1997;158:2398–2404. [PubMed] [Google Scholar]
- 151.Wicovsky A., Henkler F., Salzmann S., Scheurich P., Kneitz C., Wajant H. Tumor necrosis factor receptor-associated factor-1 enhances proinflammatory TNF receptor-2 signaling and modifies TNFR1-TNFR2 cooperation. Oncogene. 2009;28:1769–1781. doi: 10.1038/onc.2009.29. [DOI] [PubMed] [Google Scholar]
- 152.Wicovsky A., Salzmann S., Roos C., Ehrenschwender M., Rosenthal T., Siegmund D., Henkler F., Gohlke F., Kneitz C., Wajant H. TNF-like weak inducer of apoptosis inhibits proinflammatory TNF receptor-1 signaling. Cell Death Differ. 2009;16:1445–1459. doi: 10.1038/cdd.2009.80. [DOI] [PubMed] [Google Scholar]
- 153.Ivanov V.N., Fodstad O., Ronai Z. Expression of ring finger-deleted TRAF2 sensitizes metastatic melanoma cells to apoptosis via up-regulation of p38, TNFalpha and suppression of NF-kappaB activities. Oncogene. 2001;20:2243–2253. doi: 10.1038/sj.onc.1204314. [DOI] [PubMed] [Google Scholar]
- 154.Kucharczak J., Simmons M.J., Fan Y., Gélinas C. To be, or not to be: NF-kappaB is the answer--role of Rel/NF-kappaB in the regulation of apoptosis. Oncogene. 2003;22:8961–8982. doi: 10.1038/sj.onc.1207230. [DOI] [PubMed] [Google Scholar]
- 155.Zhang L., Blackwell K., Altaeva A., Shi Z., Habelhah H. TRAF2 phosphorylation promotes NF-κB-dependent gene expression and inhibits oxidative stress-induced cell death. Mol. Biol. Cell. 2011;22:128–140. doi: 10.1091/mbc.e10-06-0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Orning P., Lien E. Multiple roles of caspase-8 in cell death, inflammation, and innate immunity. J. Leukoc. Biol. 2021;109:121–141. doi: 10.1002/JLB.3MR0420-305R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Karl I., Jossberger-Werner M., Schmidt N., Horn S., Goebeler M., Leverkus M., Wajant H., Giner T. TRAF2 inhibits TRAIL- and CD95L-induced apoptosis and necroptosis. Cell Death Dis. 2014;5:e1444. doi: 10.1038/cddis.2014.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Petersen S.L., Chen T.T., Lawrence D.A., Marsters S.A., Gonzalvez F., Ashkenazi A. TRAF2 is a biologically important necroptosis suppressor. Cell Death Differ. 2015;22:1846–1857. doi: 10.1038/cdd.2015.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Anany M.A., Kreckel J., Füllsack S., Rosenthal A., Otto C., Siegmund D., Wajant H. Soluble TNF-like weak inducer of apoptosis (TWEAK) enhances poly(I:C)-induced RIPK1-mediated necroptosis. Cell Death Dis. 2018;9:1084. doi: 10.1038/s41419-018-1137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chopra M., Brandl A., Siegmund D., Mottok A., Schäfer V., Biehl M., Kraus S., Bäuerlein C.A., Ritz M., Mattenheimer K., et al. Blocking TWEAK-Fn14 interaction inhibits hematopoietic stem cell transplantation-induced intestinal cell death and reduces GVHD. Blood. 2015;126:437–444. doi: 10.1182/blood-2015-01-620583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Grabinger T., Bode K.J., Demgenski J., Seitz C., Delgado M.E., Kostadinova F., Reinhold C., Etemadi N., Wilhelm S., Schweinlin M., et al. Inhibitor of Apoptosis Protein-1 Regulates Tumor Necrosis Factor-Mediated Destruction of Intestinal Epithelial Cells. Gastroenterology. 2017;152:867–879. doi: 10.1053/j.gastro.2016.11.019. [DOI] [PubMed] [Google Scholar]
- 162.Lawlor K.E., Feltham R., Yabal M., Conos S.A., Chen K.W., Ziehe S., Graß C., Zhan Y., Nguyen T.A., Hall C., et al. XIAP Loss Triggers RIPK3- and Caspase-8-Driven IL-1β Activation and Cell Death as a Consequence of TLR-MyD88-Induced cIAP1-TRAF2 Degradation. Cell Rep. 2017;20:668–682. doi: 10.1016/j.celrep.2017.06.073. [DOI] [PubMed] [Google Scholar]
- 163.Martin-Sanchez D., Fontecha-Barriuso M., Carrasco S., Sanchez-Niño M.D., Mässenhausen A.V., Linkermann A., Cannata-Ortiz P., Ruiz-Ortega M., Egido J., Ortiz A., et al. TWEAK and RIPK1 mediate a second wave of cell death during AKI. Proc. Natl. Acad. Sci. USA. 2018;115:4182–4187. doi: 10.1073/pnas.1716578115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ivanov V.N., Kehrl J.H., Ronai Z. Role of TRAF2/GCK in melanoma sensitivity to UV-induced apoptosis. Oncogene. 2000;19:933–942. doi: 10.1038/sj.onc.1203415. [DOI] [PubMed] [Google Scholar]
- 165.Dhanasekaran D.N., Reddy E.P. JNK signaling in apoptosis. Oncogene. 2008;27:6245–6251. doi: 10.1038/onc.2008.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hoeflich K.P., Yeh W.C., Yao Z., Mak T.W., Woodgett J.R. Mediation of TNF receptor-associated factor effector functions by apoptosis signal-regulating kinase-1 (ASK1) Oncogene. 1999;18:5814–5820. doi: 10.1038/sj.onc.1202975. [DOI] [PubMed] [Google Scholar]
- 167.Nagai H., Noguchi T., Takeda K., Ichijo H. Pathophysiological roles of ASK1-MAP kinase signaling pathways. J. Biochem. Mol. Biol. 2007;40:1–6. doi: 10.5483/BMBRep.2007.40.1.001. [DOI] [PubMed] [Google Scholar]
- 168.Nishitoh H., Matsuzawa A., Tobiume K., Saegusa K., Takeda K., Inoue K., Hori S., Kakizuka A., Ichijo H. ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev. 2002;16:1345–1355. doi: 10.1101/gad.992302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Nishitoh H., Saitoh M., Mochida Y., Takeda K., Nakano H., Rothe M., Miyazono K., Ichijo H. ASK1 is essential for JNK/SAPK activation by TRAF2. Mol. Cell. 1998;2:389–395. doi: 10.1016/S1097-2765(00)80283-X. [DOI] [PubMed] [Google Scholar]
- 170.Liu H., Nishitoh H., Ichijo H., Kyriakis J.M. Activation of apoptosis signal-regulating kinase 1 (ASK1) by tumor necrosis factor receptor-associated factor 2 requires prior dissociation of the ASK1 inhibitor thioredoxin. Mol. Cell Biol. 2000;20:2198–2208. doi: 10.1128/MCB.20.6.2198-2208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yoon K.W., Cho J.H., Lee J.K., Kang Y.H., Chae J.S., Kim Y.M., Kim J., Kim E.K., Kim S.E., Baik J.H., et al. CIB1 functions as a Ca(2+)-sensitive modulator of stress-induced signaling by targeting ASK1. Proc. Natl. Acad. Sci. USA. 2009;106:17389–17394. doi: 10.1073/pnas.0812259106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Cho J.H., Lee M.K., Yoon K.W., Lee J., Cho S.G., Choi E.J. Arginine methylation-dependent regulation of ASK1 signaling by PRMT1. Cell Death Differ. 2012;19:859–870. doi: 10.1038/cdd.2011.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Galan J.A., Avruch J. MST1/MST2 Protein Kinases: Regulation and Physiologic Roles. Biochemistry. 2016;55:5507–5519. doi: 10.1021/acs.biochem.6b00763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Almanza A., Carlesso A., Chintha C., Creedican S., Doultsinos D., Leuzzi B., Luís A., McCarthy N., Montibeller L., More S., et al. Endoplasmic reticulum stress signalling—From basic mechanisms to clinical applications. Febs. J. 2019;286:241–278. doi: 10.1111/febs.14608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hetz C. The unfolded protein response: Controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 2012;13:89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- 176.Hetz C., Glimcher L.H. Fine-tuning of the unfolded protein response: Assembling the IRE1alpha interactome. Mol. Cell. 2009;35:551–561. doi: 10.1016/j.molcel.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Maurel M., Chevet E., Tavernier J., Gerlo S. Getting RIDD of RNA: IRE1 in cell fate regulation. Trends Biochem. Sci. 2014;39:245–254. doi: 10.1016/j.tibs.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 178.Park K., Ikushiro H., Seo H.S., Shin K.O., Kim Y.I., Kim J.Y., Lee Y.M., Yano T., Holleran W.M., Elias P., et al. ER stress stimulates production of the key antimicrobial peptide, cathelicidin, by forming a previously unidentified intracellular S1P signaling complex. Proc. Natl. Acad. Sci. USA. 2016;113:E1334–E1342. doi: 10.1073/pnas.1504555113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wang F., Weng H., Quon M.J., Yu J., Wang J.Y., Hueber A.O., Yang P. Dominant negative FADD dissipates the proapoptotic signalosome of the unfolded protein response in diabetic embryopathy. Am. J. Physiol. Endocrinol. Metab. 2015;309:E861–E873. doi: 10.1152/ajpendo.00215.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Brown M., Strudwick N., Suwara M., Sutcliffe L.K., Mihai A.D., Ali A.A., Watson J.N., Schröder M. An initial phase of JNK activation inhibits cell death early in the endoplasmic reticulum stress response. J. Cell Sci. 2016;129:2317–2328. doi: 10.1242/jcs.179127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sullivan G.P., O’Connor H., Henry C.M., Davidovich P., Clancy D.M., Albert M.L., Cullen S.P., Martin S.J. TRAIL Receptors Serve as Stress-Associated Molecular Patterns to Promote ER-Stress-Induced Inflammation. Dev. Cell. 2020;52:714–730.e715. doi: 10.1016/j.devcel.2020.01.031. [DOI] [PubMed] [Google Scholar]
- 182.Hu P., Han Z., Couvillon A.D., Kaufman R.J., Exton J.H. Autocrine tumor necrosis factor alpha links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1alpha-mediated NF-kappaB activation and down-regulation of TRAF2 expression. Mol. Cell Biol. 2006;26:3071–3084. doi: 10.1128/MCB.26.8.3071-3084.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Mauro C., Crescenzi E., De Mattia R., Pacifico F., Mellone S., Salzano S., de Luca C., D’Adamio L., Palumbo G., Formisano S., et al. Central role of the scaffold protein tumor necrosis factor receptor-associated factor 2 in regulating endoplasmic reticulum stress-induced apoptosis. J. Biol. Chem. 2006;2811:2631–2638. doi: 10.1074/jbc.M502181200. [DOI] [PubMed] [Google Scholar]
- 184.Lalaoui N., Lindqvist L.M., Sandow J.J., Ekert P.G. The molecular relationships between apoptosis, autophagy and necroptosis. Semin. Cell Dev. Biol. 2015;39:63–69. doi: 10.1016/j.semcdb.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 185.Long J.S., Ryan K.M. New frontiers in promoting tumour cell death: Targeting apoptosis, necroptosis and autophagy. Oncogene. 2012;31:5045–5060. doi: 10.1038/onc.2012.7. [DOI] [PubMed] [Google Scholar]
- 186.Stengel S., Messner B., Falk-Paulsen M., Sommer N., Rosenstiel P. Regulated proteolysis as an element of ER stress and autophagy: Implications for intestinal inflammation. Biochim. Biophys. Acta. Mol. Cell Res. 2017;1864:2183–2190. doi: 10.1016/j.bbamcr.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 187.Su Z., Yang Z., Xu Y., Chen Y., Yu Q. Apoptosis, autophagy, necroptosis, and cancer metastasis. Mol. Cancer. 2015;14:48. doi: 10.1186/s12943-015-0321-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Pattingre S., Tassa A., Qu X., Garuti R., Liang X.H., Mizushima N., Packer M., Schneider M.D., Levine B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 189.Maiuri M.C., Zalckvar E., Kimchi A., Kroemer G. Self-eating and self-killing: Crosstalk between autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2007;8:741–752. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- 190.Pattingre S., Bauvy C., Carpentier S., Levade T., Levine B., Codogno P. Role of JNK1-dependent Bcl-2 phosphorylation in ceramide-induced macroautophagy. J. Biol. Chem. 2009;284:2719–2728. doi: 10.1074/jbc.M805920200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Castillo K., Rojas-Rivera D., Lisbona F., Caballero B., Nassif M., Court F.A., Schuck S., Ibar C., Walter P., Sierralta J., et al. BAX inhibitor-1 regulates autophagy by controlling the IRE1α branch of the unfolded protein response. Embo. J. 2011;30:4465–4478. doi: 10.1038/emboj.2011.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.He W., Wang Q., Xu J., Xu X., Padilla M.T., Ren G., Gou X., Lin Y. Attenuation of TNFSF10/TRAIL-induced apoptosis by an autophagic survival pathway involving TRAF2- and RIPK1/RIP1-mediated MAPK8/JNK activation. Autophagy. 2012;8:1811–1821. doi: 10.4161/auto.22145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lisbona F., Rojas-Rivera D., Thielen P., Zamorano S., Todd D., Martinon F., Glavic A., Kress C., Lin J.H., Walter P., et al. BAX inhibitor-1 is a negative regulator of the ER stress sensor IRE1alpha. Mol. Cell. 2009;33:679–691. doi: 10.1016/j.molcel.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Xu D., Zhao H., Jin M., Zhu H., Shan B., Geng J., Dziedzic S.A., Amin P., Mifflin L., Naito M.G., et al. Modulating TRADD to restore cellular homeostasis and inhibit apoptosis. Nature. 2020;587:133–138. doi: 10.1038/s41586-020-2757-z. [DOI] [PubMed] [Google Scholar]
- 195.Palumbo C., De Luca A., Rosato N., Forgione M., Rotili D., Caccuri A.M. c-Jun N-terminal kinase activation by nitrobenzoxadiazoles leads to late-stage autophagy inhibition. J. Transl. Med. 2016;14:37. doi: 10.1186/s12967-016-0796-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Schultheiss U., Püschner S., Kremmer E., Mak T.W., Engelmann H., Hammerschmidt W., Kieser A. TRAF6 is a critical mediator of signal transduction by the viral oncogene latent membrane protein 1. Embo. J. 2001;20:5678–5691. doi: 10.1093/emboj/20.20.5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Münz C. Latency and lytic replication in Epstein-Barr virus-associated oncogenesis. Nat. Rev. Microbiol. 2019;17:691–700. doi: 10.1038/s41579-019-0249-7. [DOI] [PubMed] [Google Scholar]
- 198.Tsang C.M., Lui V.W.Y., Bruce J.P., Pugh T.J., Lo K.W. Translational genomics of nasopharyngeal cancer. Semin. Cancer Biol. 2020;61:84–100. doi: 10.1016/j.semcancer.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 199.Devergne O., Hatzivassiliou E., Izumi K.M., Kaye K.M., Kleijnen M.F., Kieff E., Mosialos G. Association of TRAF1, TRAF2, and TRAF3 with an Epstein-Barr virus LMP1 domain important for B-lymphocyte transformation: Role in NF-kappaB activation. Mol. Cell Biol. 1996;16:7098–7108. doi: 10.1128/MCB.16.12.7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Izumi K.M., Kaye K.M., Kieff E.D. The Epstein-Barr virus LMP1 amino acid sequence that engages tumor necrosis factor receptor associated factors is critical for primary B lymphocyte growth transformation. Proc. Natl. Acad. Sci. USA. 1997;94:1447–1452. doi: 10.1073/pnas.94.4.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Arcipowski K.M., Stunz L.L., Graham J.P., Kraus Z.J., Vanden Bush T.J., Bishop G.A. Molecular mechanisms of TNFR-associated factor 6 (TRAF6) utilization by the oncogenic viral mimic of CD40, latent membrane protein 1 (LMP1) J. Biol. Chem. 2011;286:9948–9955. doi: 10.1074/jbc.M110.185983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Busch L.K., Bishop G.A. Multiple carboxyl-terminal regions of the EBV oncoprotein, latent membrane protein 1, cooperatively regulate signaling to B lymphocytes via TNF receptor-associated factor (TRAF)-dependent and TRAF-independent mechanisms. J. Immunol. 2001;167:5805–5813. doi: 10.4049/jimmunol.167.10.5805. [DOI] [PubMed] [Google Scholar]
- 203.Soni V., Cahir-McFarland E., Kieff E. LMP1 TRAFficking activates growth and survival pathways. Adv. Exp. Med. Biol. 2007;597:173–187. doi: 10.1007/978-0-387-70630-6_14. [DOI] [PubMed] [Google Scholar]
- 204.Graham J.P., Arcipowski K.M., Bishop G.A. Differential B-lymphocyte regulation by CD40 and its viral mimic, latent membrane protein 1. Immunol. Rev. 2010;237:226–248. doi: 10.1111/j.1600-065X.2010.00932.x. [DOI] [PubMed] [Google Scholar]
- 205.Xie P., Hostager B.S., Bishop G.A. Requirement for TRAF3 in signaling by LMP1 but not CD40 in B lymphocytes. J. Exp. Med. 2004;199:661–671. doi: 10.1084/jem.20031255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Luftig M., Prinarakis E., Yasui T., Tsichritzis T., Cahir-McFarland E., Inoue J., Nakano H., Mak T.W., Yeh W.C., Li X., et al. Epstein-Barr virus latent membrane protein 1 activation of NF-kappaB through IRAK1 and TRAF6. Proc. Natl. Acad. Sci. USA. 2003;100:15595–15600. doi: 10.1073/pnas.2136756100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Chung G.T., Lou W.P., Chow C., To K.F., Choy K.W., Leung A.W., Tong C.Y., Yuen J.W., Ko C.W., Yip T.T., et al. Constitutive activation of distinct NF-κB signals in EBV-associated nasopharyngeal carcinoma. J. Pathol. 2013;231:311–322. doi: 10.1002/path.4239. [DOI] [PubMed] [Google Scholar]
- 208.Eliopoulos A.G., Blake S.M., Floettmann J.E., Rowe M., Young L.S. Epstein-Barr virus-encoded latent membrane protein 1 activates the JNK pathway through its extreme C terminus via a mechanism involving TRADD and TRAF2. J. Virol. 1999;73:1023–1035. doi: 10.1128/JVI.73.2.1023-1035.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Greenfeld H., Takasaki K., Walsh M.J., Ersing I., Bernhardt K., Ma Y., Fu B., Ashbaugh C.W., Cabo J., Mollo S.B., et al. TRAF1 Coordinates Polyubiquitin Signaling to Enhance Epstein-Barr Virus LMP1-Mediated Growth and Survival Pathway Activation. PLoS Pathog. 2015;11:e1004890. doi: 10.1371/journal.ppat.1004890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Fries K.L., Miller W.E., Raab-Traub N. The A20 protein interacts with the Epstein-Barr virus latent membrane protein 1 (LMP1) and alters the LMP1/TRAF1/TRADD complex. Virology. 1999;264:159–166. doi: 10.1006/viro.1999.9980. [DOI] [PubMed] [Google Scholar]
- 211.Laherty C.D., Hu H.M., Opipari A.W., Wang F., Dixit V.M. The Epstein-Barr virus LMP1 gene product induces A20 zinc finger protein expression by activating nuclear factor kappa B. J. Biol. Chem. 1992;267:24157–24160. doi: 10.1016/S0021-9258(18)35741-7. [DOI] [PubMed] [Google Scholar]
- 212.Gewurz B.E., Towfic F., Mar J.C., Shinners N.P., Takasaki K., Zhao B., Cahir-McFarland E.D., Quackenbush J., Xavier R.J., Kieff E. Genome-wide siRNA screen for mediators of NF-κB activation. Proc. Natl. Acad. Sci. USA. 2012;109:2467–2472. doi: 10.1073/pnas.1120542109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Grech A.P., Amesbury M., Chan T., Gardam S., Basten A., Brink R. TRAF2 differentially regulates the canonical and noncanonical pathways of NF-kappaB activation in mature B cells. Immunity. 2004;21:629–642. doi: 10.1016/j.immuni.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 214.Brown K.D., Hostager B.S., Bishop G.A. Differential signaling and tumor necrosis factor receptor-associated factor (TRAF) degradation mediated by CD40 and the Epstein-Barr virus oncoprotein latent membrane protein 1 (LMP1) J. Exp. Med. 2001;193:943–954. doi: 10.1084/jem.193.8.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Hostager B.S., Haxhinasto S.A., Rowland S.L., Bishop G.A. Tumor necrosis factor receptor-associated factor 2 (TRAF2)-deficient B lymphocytes reveal novel roles for TRAF2 in CD40 signaling. J. Biol. Chem. 2003;278:45382–45390. doi: 10.1074/jbc.M306708200. [DOI] [PubMed] [Google Scholar]
- 216.Graham J.P., Moore C.R., Bishop G.A. Roles of the TRAF2/3 binding site in differential B cell signaling by CD40 and its viral oncogenic mimic, LMP1. J. Immunol. 2009;183:2966–2973. doi: 10.4049/jimmunol.0900442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Calderwood M.A., Venkatesan K., Xing L., Chase M.R., Vazquez A., Holthaus A.M., Ewence A.E., Li N., Hirozane-Kishikawa T., Hill D.E., et al. Epstein-Barr virus and virus human protein interaction maps. Proc. Natl. Acad. Sci. USA. 2007;104:7606–7611. doi: 10.1073/pnas.0702332104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Hagemeier S.R., Barlow E.A., Kleman A.A., Kenney S.C. The Epstein-Barr virus BRRF1 protein, Na, induces lytic infection in a TRAF2- and p53-dependent manner. J. Virol. 2011;85:4318–4329. doi: 10.1128/JVI.01856-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Annunziata C.M., Davis R.E., Demchenko Y., Bellamy W., Gabrea A., Zhan F., Lenz G., Hanamura I., Wright G., Xiao W., et al. Frequent engagement of the classical and alternative NF-kappaB pathways by diverse genetic abnormalities in multiple myeloma. Cancer Cell. 2007;12:115–130. doi: 10.1016/j.ccr.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Demchenko Y.N., Glebov O.K., Zingone A., Keats J.J., Bergsagel P.L., Kuehl W.M. Classical and/or alternative NF-kappaB pathway activation in multiple myeloma. Blood. 2010;115:3541–3552. doi: 10.1182/blood-2009-09-243535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Jourdan M., Moreaux J., Vos J.D., Hose D., Mahtouk K., Abouladze M., Robert N., Baudard M., Rème T., Romanelli A., et al. Targeting NF-kappaB pathway with an IKK2 inhibitor induces inhibition of multiple myeloma cell growth. Br. J. Haematol. 2007;138:160–168. doi: 10.1111/j.1365-2141.2007.06629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Keats J.J., Fonseca R., Chesi M., Schop R., Baker A., Chng W.J., Van Wier S., Tiedemann R., Shi C.X., Sebag M., et al. Promiscuous mutations activate the noncanonical NF-kappaB pathway in multiple myeloma. Cancer Cell. 2007;12:131–144. doi: 10.1016/j.ccr.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Gardam S., Turner V.M., Anderton H., Limaye S., Basten A., Koentgen F., Vaux D.L., Silke J., Brink R. Deletion of cIAP1 and cIAP2 in murine B lymphocytes constitutively activates cell survival pathways and inactivates the germinal center response. Blood. 2011;117:4041–4051. doi: 10.1182/blood-2010-10-312793. [DOI] [PubMed] [Google Scholar]
- 224.Liu J., Hideshima T., Xing L., Wang S., Zhou W., Samur M.K., Sewastianik T., Ogiya D., An G., Gao S., et al. ERK signaling mediates resistance to immunomodulatory drugs in the bone marrow microenvironment. Sci. Adv. 2021;7:eabg2697. doi: 10.1126/sciadv.abg2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Rahal R., Frick M., Romero R., Korn J.M., Kridel R., Chan F.C., Meissner B., Bhang H.E., Ruddy D., Kauffmann A., et al. Pharmacological and genomic profiling identifies NF-κB-targeted treatment strategies for mantle cell lymphoma. Nat. Med. 2014;20:87–92. doi: 10.1038/nm.3435. [DOI] [PubMed] [Google Scholar]
- 226.Compagno M., Lim W.K., Grunn A., Nandula S.V., Brahmachary M., Shen Q., Bertoni F., Ponzoni M., Scandurra M., Califano A., et al. Mutations of multiple genes cause deregulation of NF-kappaB in diffuse large B-cell lymphoma. Nature. 2009;459:717–721. doi: 10.1038/nature07968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Xu P.P., Zhong H.J., Huang Y.H., Gao X.D., Zhao X., Shen Y., Cheng S., Huang J.Y., Chen S.J., Wang L., et al. B-cell Function Gene Mutations in Diffuse Large B-cell Lymphoma: A Retrospective Cohort Study. EBioMedicine. 2017;16:106–114. doi: 10.1016/j.ebiom.2017.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.van Galen J.C., Muris J.J., Giroth C.P., Vos W., Ossenkoppele G.J., Meijer C.J., Oudejans J.J. Expression of TNF-receptor associated factor 2 correlates with poor progression-free survival time in ABC-like primary nodal diffuse large B-cell lymphomas. Histopathology. 2008;52:578–584. doi: 10.1111/j.1365-2559.2008.02970.x. [DOI] [PubMed] [Google Scholar]
- 229.Fontan L., Goldstein R., Casalena G., Durant M., Teater M.R., Wilson J., Phillip J., Xia M., Shah S., Us I., et al. Identification of MALT1 feedback mechanisms enables rational design of potent antilymphoma regimens for ABC-DLBCL. Blood. 2021;137:788–800. doi: 10.1182/blood.2019004713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Mo Z., Wood S., Namiranian S., Mizukoshi R., Weng S., Jang I.S., Fontanillo C., Baughman J.M., Silva-Torres A., Slade M., et al. Deciphering the mechanisms of CC-122 resistance in DLBCL via a genome-wide CRISPR screen. Blood Adv. 2021;5:2027–2039. doi: 10.1182/bloodadvances.2020003431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Xie P., Stunz L.L., Larison K.D., Yang B., Bishop G.A. Tumor necrosis factor receptor-associated factor 3 is a critical regulator of B cell homeostasis in secondary lymphoid organs. Immunity. 2007;27:253–267. doi: 10.1016/j.immuni.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Moore C.R., Liu Y., Shao C., Covey L.R., Morse H.C., 3rd, Xie P. Specific deletion of TRAF3 in B lymphocytes leads to B-lymphoma development in mice. Leukemia. 2012;26:1122–1127. doi: 10.1038/leu.2011.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Zapata J.M., Krajewska M., Morse H.C., 3rd, Choi Y., Reed J.C. TNF receptor-associated factor (TRAF) domain and Bcl-2 cooperate to induce small B cell lymphoma/chronic lymphocytic leukemia in transgenic mice. Proc. Natl. Acad. Sci. USA. 2004;101:16600–16605. doi: 10.1073/pnas.0407541101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Pérez-Chacón G., Llobet D., Pardo C., Pindado J., Choi Y., Reed J.C., Zapata J.M. TNFR-associated factor 2 deficiency in B lymphocytes predisposes to chronic lymphocytic leukemia/small lymphocytic lymphoma in mice. J. Immunol. 2012;189:1053–1061. doi: 10.4049/jimmunol.1200814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Moon S.W., Son H.J., Choi E.J., Yoo N.J., Lee S.H. Brief Research Report Regional Difference in TRAF2 and TRAF3 Gene Mutations in Colon Cancers. Pathol. Oncol. Res. 2021;27:625438. doi: 10.3389/pore.2021.625438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Schürch C., Riether C., Matter M.S., Tzankov A., Ochsenbein A.F. CD27 signaling on chronic myelogenous leukemia stem cells activates Wnt target genes and promotes disease progression. J. Clin. Investig. 2012;122:624–638. doi: 10.1172/JCI45977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Park Y.H. The nuclear factor-kappa B pathway and response to treatment in breast cancer. Pharmacogenomics. 2017;18:1697–1709. doi: 10.2217/pgs-2017-0044. [DOI] [PubMed] [Google Scholar]
- 238.Velloso F.J., Bianco A.F., Farias J.O., Torres N.E., Ferruzo P.Y., Anschau V., Jesus-Ferreira H.C., Chang T.H., Sogayar M.C., Zerbini L.F., et al. The crossroads of breast cancer progression: Insights into the modulation of major signaling pathways. Onco. Targets Ther. 2017;10:5491–5524. doi: 10.2147/OTT.S142154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Durand J.K., Zhang Q., Baldwin A.S. Roles for the IKK-Related Kinases TBK1 and IKKε in Cancer. Cells. 2018;7:139. doi: 10.3390/cells7090139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Shen R.R., Zhou A.Y., Kim E., Lim E., Habelhah H., Hahn W.C. IκB kinase ε phosphorylates TRAF2 to promote mammary epithelial cell transformation. Mol. Cell. Biol. 2012;32:4756–4768. doi: 10.1128/MCB.00468-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Peramuhendige P., Marino S., Bishop R.T., de Ridder D., Khogeer A., Baldini I., Capulli M., Rucci N., Idris A.I. TRAF2 in osteotropic breast cancer cells enhances skeletal tumour growth and promotes osteolysis. Sci. Rep. 2018;8:39. doi: 10.1038/s41598-017-18327-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Papanastasiou A.D., Sirinian C., Kalofonos H.P. Identification of novel human receptor activator of nuclear factor-kB isoforms generated through alternative splicing: Implications in breast cancer cell survival and migration. Breast Cancer Res. 2012;14:R112. doi: 10.1186/bcr3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Sirinian C., Papanastasiou A.D., Schizas M., Spella M., Stathopoulos G.T., Repanti M., Zarkadis I.K., King T.A., Kalofonos H.P. RANK-c attenuates aggressive properties of ER-negative breast cancer by inhibiting NF-κB activation and EGFR signaling. Oncogene. 2018;37:5101–5114. doi: 10.1038/s41388-018-0324-y. [DOI] [PubMed] [Google Scholar]
- 244.Sun L.L., Wang J., Zhao Z.J., Liu N., Wang A.L., Ren H.Y., Yang F., Diao K.X., Fu W.N., Wan E.H., et al. Suppressive role of miR-502-5p in breast cancer via downregulation of TRAF2. Oncol. Rep. 2014;31:2085–2092. doi: 10.3892/or.2014.3105. [DOI] [PubMed] [Google Scholar]
- 245.Jiang L., Yu L., Zhang X., Lei F., Wang L., Liu X., Wu S., Zhu J., Wu G., Cao L., et al. miR-892b Silencing Activates NF-κB and Promotes Aggressiveness in Breast Cancer. Cancer Res. 2016;76:1101–1111. doi: 10.1158/0008-5472.CAN-15-1770. [DOI] [PubMed] [Google Scholar]
- 246.Ma H.Y., Li Y., Yin H.Z., Yin H., Qu Y.Y., Xu Q.Y. TNFAIP8 Promotes Cisplatin Chemoresistance in Triple-Negative Breast Cancer by Repressing p53-Mediated miR-205-5p Expression. Mol. Ther. Nucleic. Acids. 2020;22:640–656. doi: 10.1016/j.omtn.2020.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 247.Liu F., Zhou J., Zhou P., Chen W., Guo F. The ubiquitin ligase CHIP inactivates NF-κB signaling and impairs the ability of migration and invasion in gastric cancer cells. Int. J. Oncol. 2015;46:2096–2106. doi: 10.3892/ijo.2015.2893. [DOI] [PubMed] [Google Scholar]
- 248.Dai H., Chen H., Xu J., Zhou J., Shan Z., Yang H., Zhou X., Guo F. The ubiquitin ligase CHIP modulates cellular behaviors of gastric cancer cells by regulating TRAF2. Cancer Cell Int. 2019;19:132. doi: 10.1186/s12935-019-0832-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Yang H.J., Youn H., Seong K.M., Jin Y.W., Kim J., Youn B. Phosphorylation of ribosomal protein S3 and antiapoptotic TRAF2 protein mediates radioresistance in non-small cell lung cancer cells. J. Biol. Chem. 2013;288:2965–2975. doi: 10.1074/jbc.M112.385989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Vredevoogd D.W., Kuilman T., Ligtenberg M.A., Boshuizen J., Stecker K.E., de Bruijn B., Krijgsman O., Huang X., Kenski J.C.N., Lacroix R., et al. Augmenting Immunotherapy Impact by Lowering Tumor TNF Cytotoxicity Threshold. Cell. 2019;178:585–599.e515. doi: 10.1016/j.cell.2019.06.014. [DOI] [PubMed] [Google Scholar]
- 251.Litchfield K., Reading J.L., Puttick C., Thakkar K., Abbosh C., Bentham R., Watkins T.B.K., Rosenthal R., Biswas D., Rowan A., et al. Meta-analysis of tumor- and T cell-intrinsic mechanisms of sensitization to checkpoint inhibition. Cell. 2021;184:596–614.e514. doi: 10.1016/j.cell.2021.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Morrish E., Brumatti G., Silke J. Future Therapeutic Directions for Smac-Mimetics. Cells. 2020;9:406. doi: 10.3390/cells9020406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Lombrea A., Scurtu A.D., Avram S., Pavel I.Z., Turks M., Lugiņina J., Peipiņš U., Dehelean C.A., Soica C., Danciu C. Anticancer Potential of Betulonic Acid Derivatives. Int. J. Mol. Sci. 2021;22:3676. doi: 10.3390/ijms22073676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Kearney C.J., Vervoort S.J., Hogg S.J., Ramsbottom K.M., Freeman A.J., Lalaoui N., Pijpers L., Michie J., Brown K.K., Knight D.A., et al. Tumor immune evasion arises through loss of TNF sensitivity. Sci. Immunol. 2018;3:eaar3451. doi: 10.1126/sciimmunol.aar3451. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


