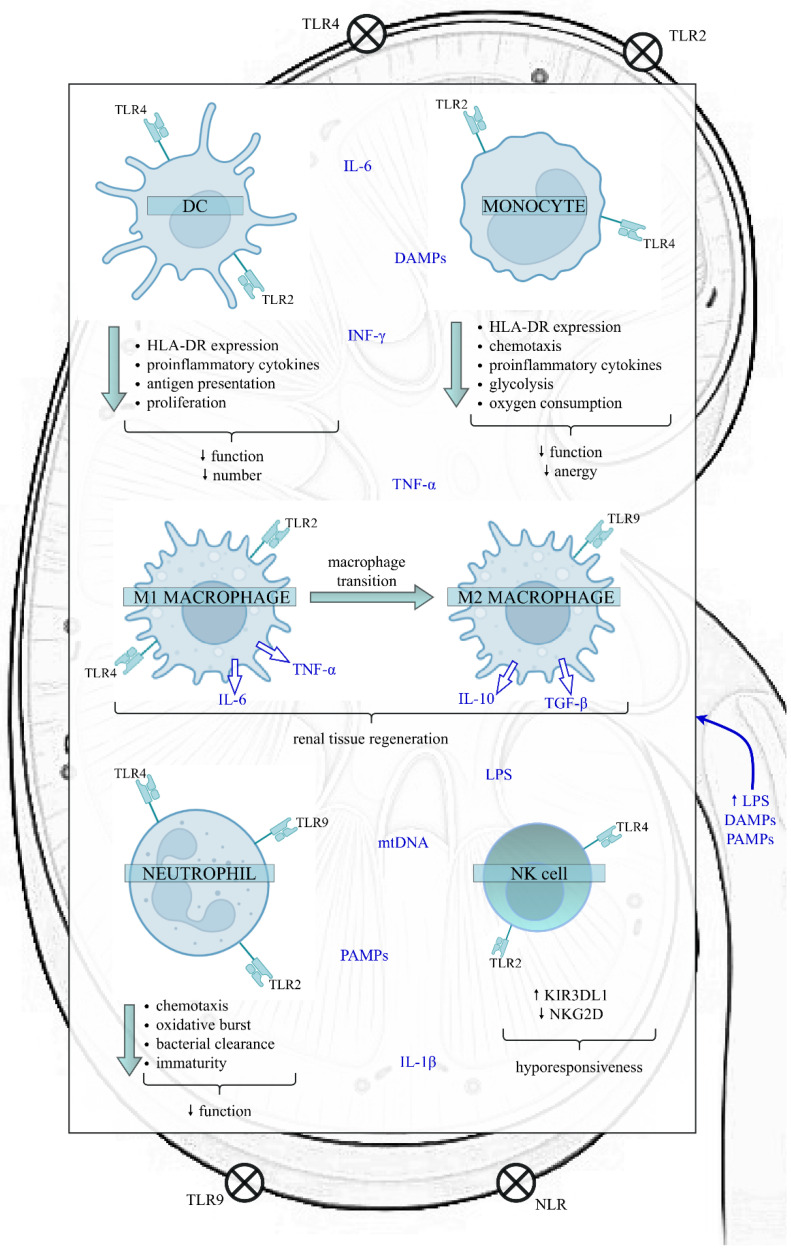
Figure 1.
The impact of sepsis on the function of the innate immune cells in the kidney. Sepsis exposes the kidney to a proinflammatory microenvironment, which impairs various kidney structures, like RTECs, Bowman’s capsular epithelium, and glomerular and endothelial cells. In the initial phase of sepsis, LPS, PAMPs and DAMPs bind to TLRs and NLRs present on the surface of both kidney and innate immune cells, initiating a downstream cascade of signals that results in the synthesis and release of proinflammatory cytokines. However, as sepsis continues, the innate immune response is altered. The above alterations include the reduced function and number of DCs, diminished function and anergy of monocytes, the transition of M1 proinflammatory to M2 anti-inflammatory macrophages, increased proportion of immature neutrophils with a weakened role and a higher proportion of NK cells expressing the inhibitory receptor KIR3DL1. Nevertheless, data on the effects of sepsis on NK cells are insufficient. In contrast, the expression of activating membrane receptor NKG2D decreases, predisposing cells to hyporesponsiveness. Disturbances of innate immunity favour immunosuppression and can have harmful clinical consequences, such as increased susceptibility to secondary infections and viral reactivations, leading to increased mortality. Abbreviations: DC—dendritic cell; NK—natural killer; HLA-DR—human leukocyte antygen-DR; TLR—Toll-like receptor; NLR—NOD-like receptor; LPS—lipopolysaccharide; PAMPs—pathogen-associated molecular patterns; DAMPs—damage-associated molecular patterns; TNF-α—tumour necrosis factor alpha; IL-6—interleukin 6; IL-1β—interleukin 1 beta; IL-10—interleukin 10; TGF-β—transforming growth factor-beta; INF-ɣ—interferon gamma; mtDNA—mitochondrial DNA; KIR3DL1—Killer cell immunoglobulin-like receptor; NKG2D—NK group 2 member D receptor.