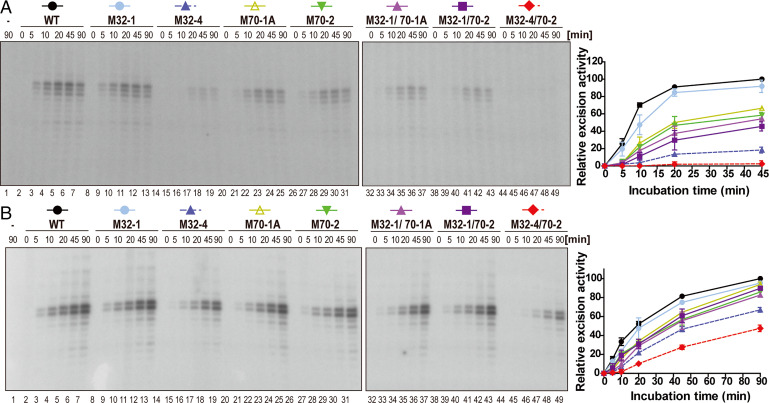
Fig. 5.
In vitro NER activity is diminished by mutations in the RPA32 and RPA70 interaction domains of XPA. (A) NER activity of XPA-deficient cell extracts with complemented with WT and mutant XPA. A plasmid containing a site-specific AAF lesion was incubated with XP2OS cell extract and the purified XPA (50 nM) proteins for 0 to 90 min. The excision products were detected by annealing to a complementary oligonucleotide with a 4-dG overhang, which was used as a template for a fill-in reaction with [α-32P] dCTP. Quantification of the data are from two independent experiments. (B) In vitro NER activity of WT and mutant XPA using purified NER proteins. Assays were conducted as in A, except that 20 nM XPA was used with together purified XPC-RAD23B (5 nM), TFIIH (10 nM), RPA (42 nM), XPG (27 nM), and XPF-ERCC1 (13 nM) proteins.