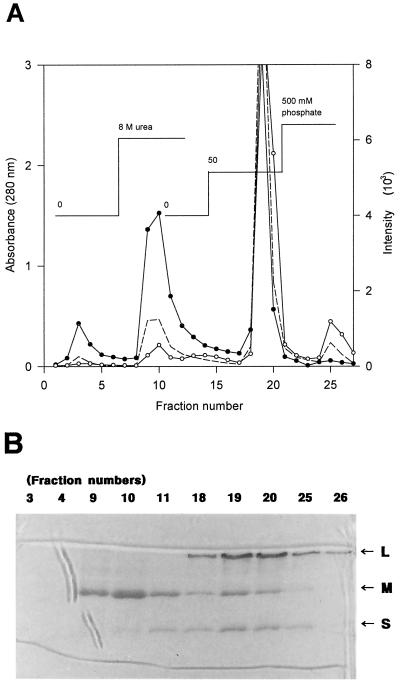
FIG. 7.
(A) Hydroxyapatite column chromatography of active CO-DH. Active purified CO-DH was applied to a hydroxyapatite column (1 by 3 cm) and eluted successively with standard buffer, standard buffer containing 8 M urea, 8 M urea-containing 50 mM phosphate buffer, and 8 M urea-containing 500 mM phosphate buffer under 15 cm of hydrostatic pressure as described in Materials and Methods. Protein was monitored by absorbance at 280 nm (–––). Cofactors were analyzed by fluorescence of emission at 460 nm after excitation at 360 nm and emission at 525 nm after excitation at 370 nm for molybdopterin (○) and FAD (●), respectively. (B) CO-DH subunits in fractions from hydroxyapatite chromatography. Denaturing PAGE of CO-DH subunits present in fractions obtained from hydroxyapatite chromatography was carried out on a 12.5% polyacrylamide gel in the presence of 0.1% SDS following the method of Laemmli (24). Twenty microliters each of several fractions eluted with standard buffer (fractions 3 and 4), standard buffer containing 8 M urea (fractions 9, 10, and 11), 50 mM phosphate buffer containing 8 M urea (fractions 18, 19, and 20), and 500 mM phosphate buffer containing 8 M urea (fractions 25 and 26) were analyzed together with active purified enzyme. Arrows indicate large (L), medium (M), and small (S) subunits of CO-DH.