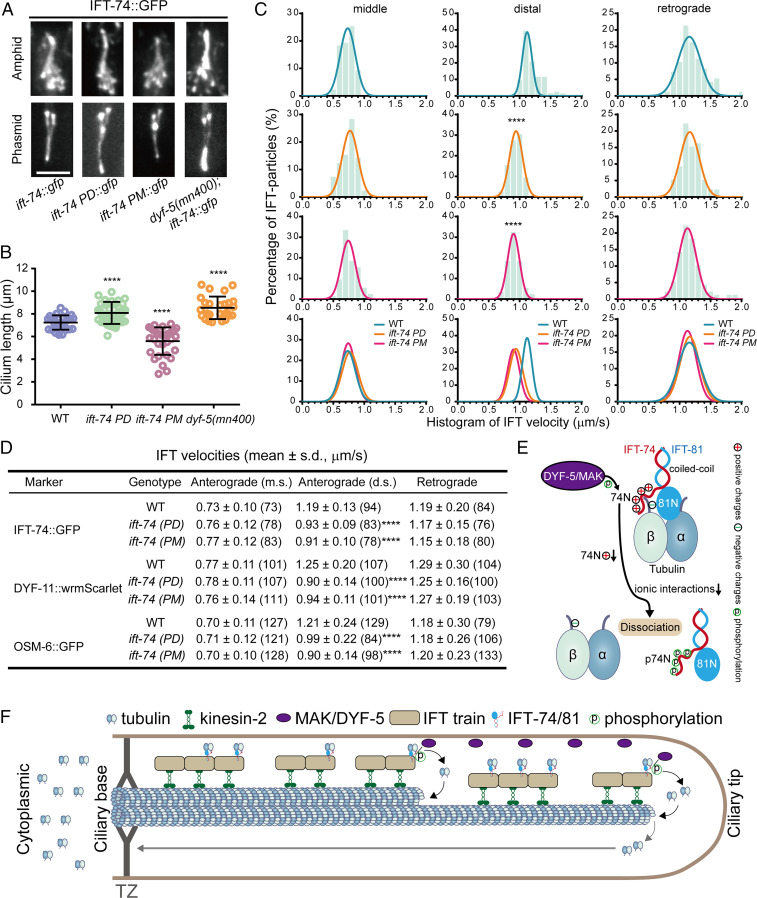
Fig. 4.
Phosphorylation of IFT-74 controls cilium length via regulating intraciliary tubulin transport. (A) Amphid (Top) and phasmid (Bottom) cilia morphology in ift-74 PM and PD mutants in comparison with WT (N2) and dyf-5 null mutant (mn400) animals. Cilia were imaged with GFP-tagged WT or mutated IFT-74; IFT-74 PD::GFP and IFT-74 PM::GFP were tracked, and the IFT-74::GFP was tracked in the WT animals or dyf-5(mn400) null mutant worms. (Scale bar: 5 μm.) (B) Cilium length in WT, dyf-5(mn400), and ift-74 PM/PD animals (mean ± SD; n = 30; ****P < 0.0001). IFT-74::GFP was used as the cilium marker. (C) Histogram of IFT velocities in WT and ift-74 mutant animals. (Left) Anterograde IFT along the middle segments. (Middle) Anterograde IFT along the distal segments. (Right) Retrograde IFT. IFT-74::GFP was used as the IFT marker for the measurement. Each plot was fit by a Gaussian distribution. Comparisons were performed between the WT and mutants. ****P < 0.0001. (D) Statistics of IFT velocities of WT and mutant animals measured independently using IFT-74::GFP, DYF-11::wrmScarlet, and OSM-6::GFP as ciliary IFT markers. m.s.: middle segment; d.s.: distal segment; ****P < 0.0001. The n values are given in the parentheses. PM: phospho-mimic mutant; PD: phospho-dead mutant. (E) Model representation of DYF-5/MAK–induced IFT-74/81–tubulin dissociation via IFT-74N phosphorylation. (F) Model illustrating the regulation of intraciliary tubulin transport by DYF-5/MAK–mediated IFT-74 phosphorylation. IFT-74/81 binds and transports tubulin toward ciliary middle and tip, where DYF-5/MAK phosphorylates the IFT-74 tubulin-binding region to release bound tubulin for ciliary construction and maintenance. TZ: transition zone.