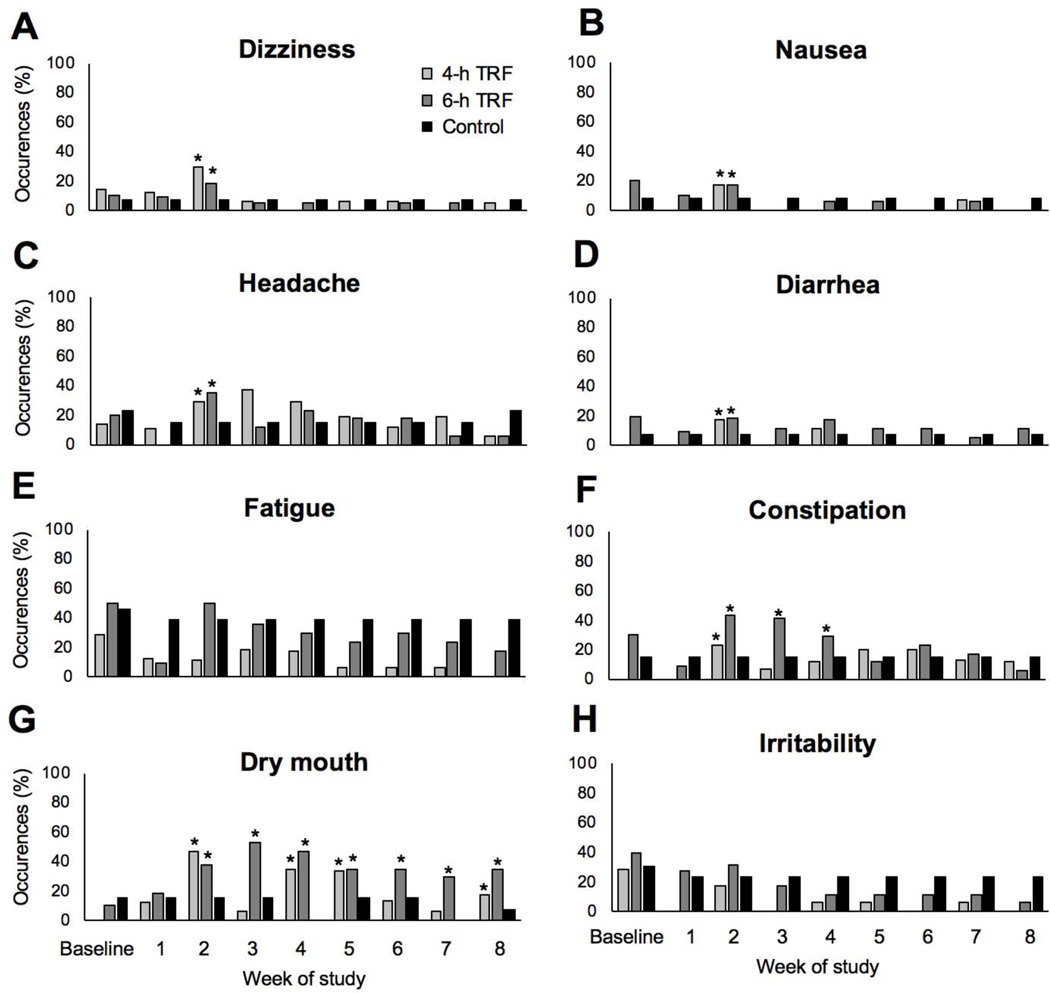
Figure 6. Adverse events.
A) Percent occurrences of dizziness at each week of the study.
B) Percent occurrences of nausea at each week of the study.
C) Percent occurrences of headache at each week of the study.
D) Percent occurrences of diarrhea at each week of the study.
E) Percent occurrences of fatigue at each week of the study.
F) Percent occurrences of constipation at each week of the study.
G) Percent occurrences of dry mouth at each week of the study.
H) Percent occurrences of irritability at each week of the study.
All values reported as percent occurrences at each week of the study. * P < 0.05, percent occurrences significantly different from controls at each time point by ANCOVA.