Fig. 3.
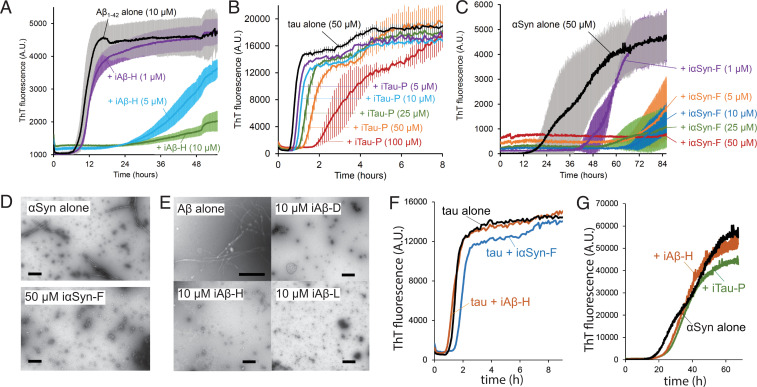
Inhibitors prevent amyloid aggregation by capping fibril ends. (A–C) Thioflavin T aggregation kinetics assays for Aβ (A), tau (B), and αSyn (C). Each amyloid protein was aggregated alone and in the presence of increasing concentrations of inhibitor, resulting in reduction in the rate of aggregation or complete abolition of aggregation. (A) The aggregation of Aβ1–42 (10 µM monomer) with the inhibitor iAβ-H. (B) Aggregation of αSyn (50 µM monomer) with inhibitor iαSyn-F nearly eliminates measured aggregation, even at substoichiometric ratios. (C) Tau k18+ (50 µM monomer) with the inhibitor iTau-P (100 µM) produces a fourfold increase in aggregation lag time. (D) Transmission electron micrographs (TEMs) of αSyn (50 µM) aggregated in the absence (Top) or presence (Bottom) of iαSyn-F show the inhibitor prevents the formation of fibrillar aggregates. (Scale bar, 50 nm.) (E) TEM images of Aβ1–42 alone (10 µM) reveal numerous fibrils, whose growth is prevented by the addition of equimolar amounts of iAβ-D, iAβ-H, and iAβ-L. (Scale bar, 100 nm.) (F) Both iαSyn-F and iAβ-H show little effect on tau k18+ aggregation (50 µM k18+ monomer, 50 µM inhibitors). Likewise, iTau-N and iAβ-H have minimal influence on αSyn aggregation (50 µM αSyn monomer, 50 µM inhibitors). All ThT experiments were performed with n = 3 experimental replicates.