Abstract
Circular RNAs (circRNAs) are potential biomarkers owing to their stability, tissue specificity, and abundance. This study aimed to evaluate the clinical significance of hsa_circ_0003570 expression and to investigate its potential as a biomarker in hepatocellular carcinoma (HCC). We evaluated hsa_circ_0003570 expression in 121 HCC tissue samples, its association with clinicopathological characteristics, and overall and progression-free survival. Hsa_circ_0003570 expression was downregulated in HCC tissues. Low hsa_circ_0003570 expression was more common in tumors larger than 5 cm (odds ratio (OR), 6.369; 95% confidence interval (CI), 2.725–14.706; p < 0.001), vessel invasion (OR, 5.128; 95% CI, 2.288–11.494; p < 0.001); advanced tumor-node metastasis stage (III/IV; OR, 4.082; 95% CI, 1.866–8.929; p < 0.001); higher Barcelona Clinic Liver Cancer stage (B/C; OR, 3.215; 95% CI, 1.475–6.993; p = 0.003); and higher AFP (>200 ng/mL; OR, 2.475; 95% CI, 1.159–5.291; p = 0.018). High hsa_circ_0003570 expression was an independent prognostic factor for overall survival (hazard ratio (HR), 0.541; 95% confidence interval (CI), 0.327–0.894; p = 0.017) and progression-free survival (HR, 0.633; 95% CI, 0.402–0.997; p = 0.048). Hsa_circ_0003570 is a potential prognostic biomarker in patients with HCC, and further validation of hsa_circ_0003570 is needed.
Keywords: circular RNA, epigenetics, hepatocellular carcinoma, survival, progression, biomarker, prognosis
1. Introduction
Circular RNAs (circRNAs) are single-stranded, covalently closed RNA molecules produced from pre-mRNAs through backsplicing. Advances in RNA sequencing and bioinformatics tools have enabled the discovery of various circRNAs and their functions [1]. CircRNAs have recently been identified as microRNA sponges [2], modulators of transcription [3], and protein-binding decoys or sponges. Moreover, circRNAs are known to function as hallmarks of cancer that can explain the transition of normal cells to cancerous cells [4]. CircRNAs are involved in various functions, including sustaining growth signaling, evading growth inhibitors, resisting apoptosis, uncontrolled replicative immortality, promoting angiogenesis, and activating invasion and metastasis [5]. Therefore, circRNAs have been shown to be related to tumorigenesis [6], epithelial–mesenchymal transition [7], and tumor progression in various cancers.
In addition to being cancer hallmarks, circRNAs have also been shown to be highly stable and can be found in exosomes, saliva, urine, and plasma [8,9]. Thus, circRNAs are considered good cancer biomarker candidates.
Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer and the most common cause of death in people with cirrhosis [10]. The prognosis for HCC is poor because it tends to be diagnosed at an advanced stage. It is often asymptomatic until the tumor becomes large enough to tighten the liver capsule or compress adjacent organs or vessels with nerves, causing pain. The other cause of poor prognosis is that the state of the underlying liver disease limits treatment options and has an adverse effect on results, regardless of the HCC stage. Due to the immunosuppressive tumor microenvironment of HCC, new treatment targets and strategies have been proposed [11], and a new biomarker for predicting prognosis is necessary.
For investigating circRNAs as a biomarker in HCC, hsa_circ_00033570 was previously reported and analyzed in HCC tissues [12]. A previous study showed that the expression level of hsa_circ_0003570 was associated with clinicopathological characteristics. Therefore, we investigated the clinical significance and application of hsa_circ_0003570 as a biomarker in HCC.
2. Materials and Methods
2.1. Patients and Tissue Samples
This study included 162 patients with HCC who underwent a diagnostic biopsy or surgical resection at a single center between March 2015 and August 2016, and who have previously been studied [13]. We excluded patients who had previously been treated for HCC (n = 30) and those lost to follow-up (n = 9). Two patients were not checked for target circRNAs due to a shortage of tissue samples. Finally, 121 patients were included in the analysis. The median follow-up period was 24.5 months, ranging from 0.7 to 69.8 months.
The patients were monitored every three months using liver dynamic computed tomography (CT) or gadoxetic acid disodium–enhanced liver magnetic resonance imaging. HCC recurrence was recognized if a tumor exceeded 1 cm and showed contrast enhancement in the arterial phase and washout in the portal or delayed phase. Response Evaluation Criteria in Solid Tumors (version 1.1) was used to evaluate tumor response. We defined overall survival as the time between the date of initial HCC diagnosis, and either the date of death from any cause or the date of last contact with the patient. Progression-free survival was defined as the time between the initial date of HCC diagnosis, and either the first event of recurrence or progression or until death from any cause.
The tissue specimens for tumors and adjacent nontumor tissues were immediately stored at 4 °C for 24 h in RNAlater reagent (Ambion; Life Technologies, Carlsbad, CA, USA) and then stored at −80 °C. We collected patients’ clinicopathological data, including age, sex, etiology of liver disease, Child–Tucotte–Pugh (CTP) class, laboratory findings, α-fetoprotein (AFP) level, tumor size and number, presence of macrovascular invasion, and tumor stage. The tumor-node metastasis (TNM) stage, based on the criteria of the American Joint Committee on Cancer, 8th edition, and Barcelona Clinic Liver Cancer (BCLC) stage, was adopted. This study was approved by the institutional review board (KNUH-2014-04-056-001) and was conducted in accordance with the ethical guidelines of the 1975 Declaration of Helsinki. Written informed consent was obtained from all patients prior to sample collection.
2.2. Extraction of Total RNA and Synthesis of cDNA
Total RNA was extracted from the frozen tissues using QIAzol Lysis Reagent (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. Total RNA samples were verified for concentration and purity using NanoPhotometer N60 (Implen NanoPhotometer, Westlake Village, CA, USA). Synthesis of cDNA was reverse transcribed using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) following the manufacturer’s instructions.
2.3. Quantitative Real-Time Polymerase Chain Reaction
Quantitative real-time polymerase chain reaction (qRT-PCR) was performed with the SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA) following the manufacturer’s instructions. For circular RNA expression analysis, the primers of hsa_circ_0003570 were designed, including a gap junction of circular RNA. The primers’ sequences of hsa_circ_0003570 were 5′- CAA GAT GGC ACA GCA GCA CAC GC -3′ (forward) and 5′- ATG CTG GTG CTC GGT TGG TC -3′. The primers’ sequences of lyceraldehyde 3-phosphate dehydrogenase (GAPDH), as a normalizer, were 5′- GGA AGG TGA AGG TCG GAG TC -3′ (forward) and 5′- GTT GAG GTC AAT GAA GGG GTC -3′. All of the primers were synthesized by Bionics (Seoul, Korea). qRT-PCR was performed in triplicate and amplification of hsa_circ_0003570 was confirmed via melt curve analysis. The relative expression results from the qRT-PCR were calculated with the 2−ΔΔCt method.
2.4. Statistical Analysis
Categorical data are expressed as numbers (%), and numerical data are expressed as the mean and standard deviation for normally distributed data. For non-normally distributed data, the data are expressed as medians with interquartile ranges. To analyze the differences in hsa_circ_0003570 expression between the tumor and adjacent nontumor tissues, a paired t-test was used. To compare the clinicopathological characteristics between the two groups according to the hsa_circ_0003570 expression, we used the chi-square or Fisher’s exact probability test. Using the Kaplan–Meier method and log-rank test, we analyzed patient survival and compared survival between the groups. To identify the predictors of survival, we performed a logistic regression based on the Cox proportional hazards model. The statistical significance was set at p < 0.05. We conducted all the analyses using R statistical software 3.6.3 (the R foundation for Statistical Computing, Vienna, Austria; available at http://www.r-project.org, accessed on 5 March 2020), and the GraphPad Prism 6 program for Windows (GraphPad Software, La Jolla, CA, USA) was used to generate figures.
3. Results
3.1. Baseline Characteristics of Hepatocellular Carcinoma Patients
Table 1 presents the baseline patient characteristics. Among the 121 patients, 19 (15.7%) underwent surgical resection and 46 (38.0%) underwent radiofrequency ablation as curative treatment. The other 11 patients (9.1%) underwent transarterial chemoembolization, 14 (11.6%) started sorafenib, and 31 (25.6%) received best supportive care as noncurative treatment. Seventy-three percent of the patients had viral hepatitis as an underlying disease.
Table 1.
Baseline characteristics of enrolled patients.
| Clinical Characteristics | Total (n = 121) |
|---|---|
| Age (years) | 60.8 ± 10.6 |
| Sex | |
| Male | 104 (86.0%) |
| Female | 17 (14.0%) |
| Etiology | |
| HBV | 74 (61.2%) |
| HCV | 11 (9.1%) |
| Alcohol consumption | 32 (26.4%) |
| HBV + HCV | 2 (1.7%) |
| NASH | 2 (1.7%) |
| Tumor number | |
| Single | 67 (55.4%) |
| Multiple | 54 (44.6%) |
| Size of tumor (cm) | |
| ≤5 | 59 (48.8%) |
| >5 | 62 (51.2%) |
| Vessel invasion | |
| No | 79 (65.3%) |
| Yes | 42 (34.7%) |
| TNM stage | |
| I | 51 (42.1%) |
| II | 15 (12.4%) |
| III | 13 (10.7%) |
| IV | 42 (34.7%) |
| BCLC stage | |
| 0/A | 59 (48.8%) |
| B/C | 62 (51.2%) |
| CTP class | |
| A | 104 (86.0%) |
| B | 17 (14.0%) |
| AST (U/L) | 50.0 [29.0–74.0] * |
| ALT (U/L) | 37.0 [25.0–53.0] * |
| Total bilirubin (mg/dL) | 0.7 [0.5–1.1] * |
| Albumin (g/dL) | 3.9 [3.5–4.2] * |
| Prothrombin time (s) | 12.5 [11.8–13.2] * |
| AFP (ng/mL) | 57.9 [7.5;2376.0] * |
HBV—hepatitis B virus; HCV—hepatitis C virus; NASH—nonalcoholic steatohepatitis; TNM—tumor-node metastasis; BCLC—Barcelona Clinic Liver Cancer; CTP—Child–Turcotte–Pugh; AST—aspartate transaminase; ALT—alanine transaminase; AFP—α-fetoprotein; * median [25–75% interquartile range].
3.2. Downregulation of hsa_circ_0003570 Expression in Hepatocellular Carcinoma Tissues
Figure 1 shows that the expression of hsa_circ_0003570 in HCC tissues was lower than that in noncancerous tissues (p = 0.001).
Figure 1.
Dot plot of hsa_circ_0003570 expression in noncancerous (NC) tissue and cancer tissue in hepatocellular carcinoma. A paired t-test showed that hsa_circ_0003570 expression decreased in tumor tissue compared to that in the NC tissue (p = 0.001).
3.3. Correlation between hsa_0003570 Expression and Clinicopathological Characteristics of Hepatocellular Carcinoma Patients
The differences in patient characteristics based on the expression levels of hsa_circ_0003570 are shown in Table 2. Based on the hsa_circ_0003570 expression, all patients were classified into high-expression (≥0.0005554) or low-expression (<0.0005554) groups. The cut-off value was determined using maximally selected rank statistics in the Maxstat R package. The optimal cut-offs were defined as the expression of hsa_circ_0003570 that best separated the two groups in terms of survival. Low hsa_circ_0003570 expression was associated with tumors larger than 5 cm (odds ratio [OR], 6.369; 95% confidence interval [CI], 2.725–14.706; p < 0.001); vessel invasion (Yes; OR, 5.128; 95% CI, 2.288–11.494; p < 0.001); advanced TNM stage (III/IV; OR, 4.082; 95% CI, 1.866–8.929; p < 0.001); higher BCLC stage (B/C; OR, 3.215; 95% CI, 1.475–6.993; p = 0.003); and higher AFP (>200 ng/mL; OR, 2.475; 95% CI, 1.159–5.291; p = 0.018).
Table 2.
Clinical characteristics of patients according to hsa_circ_0003570 expression.
| Clinical Characteristics | hsa_circ_0003570 | ||
|---|---|---|---|
| Low n = 45) |
High (n = 76) |
p-Value | |
| Age (years) | 0.868 | ||
| ≤60 | 24 (53.3%) | 38 (50.0%) | |
| >60 | 21 (46.7%) | 38 (50.0%) | |
| Sex | 0.656 | ||
| Male | 40 (88.9%) | 64 (84.2%) | |
| Female | 5 (11.1%) | 12 (15.8%) | |
| Tumor number | 0.360 | ||
| Single | 22 (48.9%) | 45 (59.2%) | |
| Multiple | 23 (51.1%) | 31 (40.8%) | |
| Tumor size (cm) | < 0.001 * | ||
| ≤5 | 10 (22.2%) | 49 (64.5%) | |
| >5 | 35 (77.8%) | 27 (35.5%) | |
| Vessel invasion | < 0.001 * | ||
| No | 19 (42.2%) | 60 (78.9%) | |
| Yes | 26 (57.8%) | 16 (21.1%) | |
| TNM stage | 0.001 * | ||
| I/II | 15 (33.3%) | 51 (67.1%) | |
| III/IV | 30 (66.7%) | 25 (32.9%) | |
| BCLC stage | 0.005 * | ||
| O/A | 14 (31.1%) | 45 (59.2%) | |
| B/C | 31 (68.9%) | 31 (40.8%) | |
| CTP classification | 1.000 | ||
| A | 39 (86.7%) | 65 (85.5%) | |
| B | 6 (13.3%) | 11 (14.5%) | |
| AFP (ng/mL) | 0.030 * | ||
| ≤200 | 21 (46.7%) | 52 (68.4%) | |
| >200 | 24 (53.3%) | 24 (31.6%) | |
| Chronic hepatitis B | 0.994 | ||
| No | 18 (40.0%) | 29 (38.2%) | |
| Yes | 27 (60.0%) | 47 (61.8%) | |
TNM—tumor-node metastasis; BCLC—Barcelona Clinic Liver Cancer; CTP—Child–Turcotte–Pugh; AFP—α-fetoprotein. * p < 0.05.
3.4. Correlation between hsa_0003570 Expression and Survival of Hepatocellular Carcinoma Patients
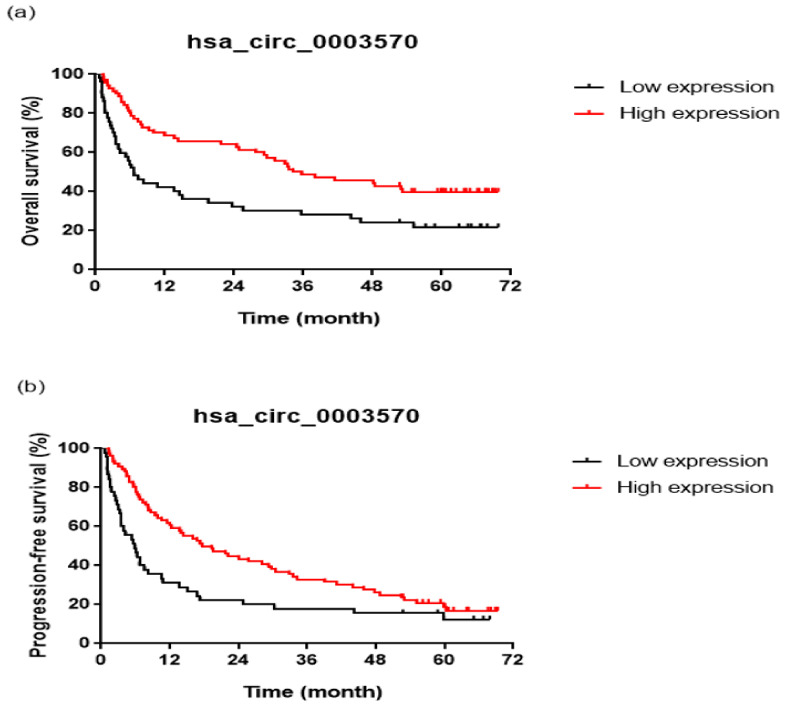
The overall survival of the patients differed significantly according to the hsa_circ_0003570 expression (Figure 2a). The cumulative 1-, 2-, and 4-year overall survival rates were 40.0%, 31.1%, and 24.4%, respectively, in the low-expression group and 68.1%, 62.7%, and 40.0%, respectively, in the high-expression group.
Figure 2.
Survival curves according to the hsa_circ_0003570 expression level. Overall survival (a) (log-rank test, p = 0.002) and progression-free survival (b) (log-rank test, p = 0.007) rates were decreased in the low hsa_circ_0003570 expression level group.
Table 3 shows the significant predictors of overall survival. Univariate analysis of prognostic factors for overall survival in patients with HCC demonstrated high hsa_circ_0003570 expression (hazard ratio [HR], 0.515; 95% CI, 0.331–0.801; p = 0.003); multiple tumors (HR, 2.408; 95% CI, 1.549–3.743; p < 0.001); AFP level > 200 ng/mL (HR, 2.938; 95% CI, 1.885–4.579; p < 0.001); poor CTP class (HR, 3.259; 95% CI, 1.881–5.648; p < 0.001); chronic hepatitis B (HR, 0.594; 95% CI, 0.383–0.922; p = 0.020); and curative treatment (HR, 0.141; 95% CI, 0.086–0.231; p < 0.001).
Table 3.
Prognostic factors for overall survival in univariable and multivariable analyses.
| Factor | Univariable Analysis | Multivariable Analysis | ||
| Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value | |
| Hsa_circ_0003570 (high expression) | 0.515 (0.331–0.801) | 0.003 * | 0.541 (0.327–0.894) | 0.017 * |
| Age (>60 years) | 1.100 (0.711–1.701) | 0.669 | 1.397 (0.820–2.381) | 0.219 |
| Sex (male) | 1.097 (0.594–2.026) | 0.766 | 1.368 (0.679–2.756) | 0.381 |
| Tumor number (multiple) | 2.408 (1.549–3.743) | <0.001 * | 0.825 (0.463–1.470) | 0.514 |
| AFP (>200 ng/mL) | 2.938 (1.885–4.579) | <0.001 * | 1.457 (0.871–2.438) | 0.152 |
| CTP classification (B vs. A) | 3.259 (1.881–5.648) | <0.001 * | 2.271 (1.122–4.595) | 0.023 * |
| Chronic hepatitis B | 0.594 (0.383–0.922) | 0.020 * | 0.809 (0.486–1.347) | 0.415 |
| Curative treatment | 0.141 (0.086–0.231) | <0.001 * | 0.171 (0.090–0.325) | <0.001 * |
CI—confidence interval; AFP—α-fetoprotein; CTP—Child–Turcotte–Pugh. * p < 0.05.
Multivariate analysis identified high hsa_circ_0003570 expression (HR, 0.541; 95% CI, 0.327–0.894; p = 0.017); poor CTP class (HR, 2.271; 95% CI, 1.122–4.595; p = 0.023); and curative treatment (HR, 0.171; 95% CI, 0.090–0.325; p < 0.001) as independent prognostic factors for overall survival.
3.5. Correlation between hsa_0003570 Expression and Progression-Free Survival of Hepatocellular Carcinoma Patients
Progression-free survival was significantly different between patients according to their hsa_circ_0003570 expression levels (Figure 2b). The cumulative 1-, 2-, and 4-year progression-free survival rates were 31.1%, 22.2%, and 15.6%, respectively, in the low-expression group, and 60.5%, 43.4%, and 26.3%, respectively, in the high-expression group.
Table 4 shows the significant predictors of progression-free survival. Univariate analysis of prognostic factors for progression-free survival in patients with HCC demonstrated high hsa_circ_0003570 expression (hazard ratio [HR], 0.580; 95% CI, 0.388–0.867; p = 0.008); multiple tumors (HR, 1.950; 95% CI, 1.316–2.889; p = 0.001); AFP level >200 ng/mL (HR, 2.767; 95% CI, 1.845–4.148; p < 0.001); poor CTP class (HR, 2.869; 95% CI, 1.676–4.911; p < 0.001); and curative treatment (HR, 0.186; 95% CI, 0.120–0.288; p < 0.001).
Table 4.
Prognostic factors for progression-free survival in univariable and multivariable analyses.
| Factor | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value | |
| Hsa_circ_0003570 (high expression) | 0.580 (0.388–0.867) | 0.008 * | 0.633 (0.402–0.997) | 0.048 * |
| Age (>60 years) | 0.887 (0.600–1.311) | 0.548 | 0.919 (0.575–1.467) | 0.723 |
| Sex (Male) | 1.216 (0.713–2.076) | 0.473 | 1.719 (0.945–3.125) | 0.076 |
| Tumor number (multiple) | 1.950 (1.316–2.889) | 0.001 * | 0.754 (0.455–1.248) | 0.272 |
| AFP (>200 ng/mL) | 2.767 (1.845–4.148) | <0.001 * | 1.569 (0.963–2.557) | 0.070 |
| CTP classification (B vs. A) | 2.869 (1.676–4.911) | <0.001 * | 2.163 (1.098–4.261) | 0.026 * |
| Chronic hepatitis B | 0.766 (0.514–1.141) | 0.190 | 0.967 (0.614–1.526) | 0.887 |
| Curative treatment | 0.186 (0.120–0.288) | <0.001 * | 0.235 (0.133–0.416) | <0.001 * |
CI—confidence interval; AFP—α-fetoprotein; CTP—Child–Turcotte–Pugh. * p < 0.05.
Multivariate analysis identified that high hsa_circ_0003570 expression (HR, 0.633; 95% CI, 0.402–0.997; p = 0.048); poor CTP class (HR, 2.163; 95% CI, 1.098–4.261; p = 0.026); and curative treatment (HR, 0.235; 95% CI, 0.133–0.416; p < 0.001) were independent prognostic factors for progression-free survival.
4. Discussion
CircRNAs are well-known potential biomarkers in cancer research because of their stability [14], tissue specificity [1], and abundance [15]. Numerous studies have reported the potential of these biomarkers in the diagnosis of various cancers [16]. CircRNAs can improve the performance of protein-based biomarkers, predict prognosis and treatment response, detect cancer early, and monitor recurrence. In HCC, some circRNAs have been reported to be associated with overall and recurrence-free survival after hepatectomy. For example, circTRIM33–12 [17], circSMARCA5 [18], and circADAMTS13 [19] act as the sponge of miRNAs to regulate HCC progression. In this study, we recruit not only early stage HCC cases treated by hepatectomy, but also advanced HCC cases beyond curative treatment, and showed the association between hsa_circ_0003570 and survival.
We also discovered that hsa_circ_0003570 was downregulated in HCC compared to noncancerous human liver tissue. Its expression level was associated with various clinicopathological characteristics, particularly tumor size, vessel invasion, TNM stage, BCLC stage, and AFP level. Low expression of hsa_circ_0003570 was associated with poor overall and progression-free survival, among other clinical variables.
Our results are consistent with those of a previous study [12]. Previously, hsa_circ_0003570 was reported to be downregulated in HCC cell lines and tissues. In that study, hsa_circ_0003570 was also associated with several clinicopathological characteristics, such as tumor size, tumor differentiation, microvascular invasion, BCLC stage, TNM stage, and AFP level.
The difference between the two studies is that we enrolled patients with advanced-stage HCC and considered the survival and progression of HCC according to the treatment modalities. To the best of our knowledge, this study is the first to discover an association between hsa_circ_0003570 and survival and progression in HCC patients. This raises the possibility of using hsa_circ_0003570 as a prognostic biomarker for HCC.
However, this study had some limitations. First, the retrospective nature of the study introduces a selection bias. We excluded 11 patients with HCC who were missing medical records due to loss to follow-up. Second, we could not acquire further pathological information, such as microvascular invasion or cell differentiation, owing to a needle biopsy performed in the advanced stage of HCC. Moreover, hsa_circ_0003570 cannot reflect the tumor heterogeneity of HCC. Therefore, it is necessary to recruit a larger number of HCC patients and validate hsa_circ_0003570 as a noninvasive biomarker using patient serum or urine. Third, we did not investigate the underlying mechanism of hsa_circ_0003570. Based on these results, we can only hypothesize that these circRNAs may act as a tumor suppressor.
5. Conclusions
In conclusion, we explored the clinical significance of hsa_circ_0003570 in patients with HCC. It was associated not only with the clinicopathological characteristics of HCC, but also with the survival and progression of HCC patients. Hsa_circ_0003570 can be a potential prognostic biomarker in patients with HCC, but further validation of hsa_circ_0003570 is needed in a future study.
Author Contributions
Conceptualization, S.Y.P. and K.H.; Formal analysis, S.Y.J.; Funding acquisition, S.Y.J. and K.H.; Investigation, G.K., Y.R.L., Y.O.K. and W.Y.T.; Resources, Y.S.H., J.R.H. and S.Y.P.; Methodology, G.K., J.G.P., M.K.K., H.W.L. and W.K.L.; Supervision, S.Y.P. and K.H.; Writing—original draft, S.Y.J., G.K.; Writing—review and editing, S.Y.P. and K.H. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Kyungpook National University Hospital (KNUH-2014-04-056-001).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the National Research Foundation (NRF) grants funded by the Korean government (grant numbers 2017M3A9G8083382, 2019R1A2C1083892, 2021R1A5A2021614, and 2019R1F1A1060878 (Ministry of Science and ICT)).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Salzman J., Chen R.E., Olsen M.N., Wang P.L., Brown P.O. Cell-type specific features of circular RNA expression. PLoS Genet. 2013;9:e1003777. doi: 10.1371/annotation/f782282b-eefa-4c8d-985c-b1484e845855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hansen T.B., Jensen T.I., Clausen B.H., Bramsen J.B., Finsen B., Damgaard C.K., Kjems J. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384–388. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 3.Memczak S., Jens M., Elefsinioti A., Torti F., Krueger J., Rybak A., Maier L., Mackowiak S.D., Gregersen L.H., Munschauer M., et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495:333–338. doi: 10.1038/nature11928. [DOI] [PubMed] [Google Scholar]
- 4.Hanahan D., Weinberg R.A. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 5.Su M., Xiao Y., Ma J., Tang Y., Tian B., Zhang Y., Li X., Wu Z., Yang D., Zhou Y., et al. Circular RNAs in Cancer: Emerging functions in hallmarks, stemness, resistance and roles as potential biomarkers. Mol. Cancer. 2019;18:90. doi: 10.1186/s12943-019-1002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guarnerio J., Bezzi M., Jeong J.C., Paffenholz S.V., Berry K., Naldini M.M., Lo-Coco F., Tay Y., Beck A.H., Pandolfi P.P. Oncogenic Role of Fusion-circRNAs Derived from Cancer-Associated Chromosomal Translocations. Cell. 2016;165:289–302. doi: 10.1016/j.cell.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 7.Conn S.J., Pillman K.A., Toubia J., Conn V.M., Salmanidis M., Phillips C.A., Roslan S., Schreiber A.W., Gregory P.A., Goodall G.J. The RNA binding protein quaking regulates formation of circRNAs. Cell. 2015;160:1125–1134. doi: 10.1016/j.cell.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 8.Li Y., Zheng Q., Bao C., Li S., Guo W., Zhao J., Chen D., Gu J., He X., Huang S. Circular RNA is enriched and stable in exosomes: A promising biomarker for cancer diagnosis. Cell Res. 2015;25:981–984. doi: 10.1038/cr.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bahn J.H., Zhang Q., Li F., Chan T.M., Lin X., Kim Y., Wong D.T., Xiao X. The landscape of microRNA, Piwi-interacting RNA, and circular RNA in human saliva. Clin. Chem. 2015;61:221–230. doi: 10.1373/clinchem.2014.230433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forner A., Llovet J.M., Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 11.Leone P., Solimando A.G., Fasano R., Argentiero A., Malerba E., Buonavoglia A., Lupo L.G., De Re V., Silvestris N., Racanelli V. The Evolving Role of Immune Checkpoint Inhibitors in Hepatocellular Carcinoma Treatment. Vaccines. 2021;9:532. doi: 10.3390/vaccines9050532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fu L., Wu S., Yao T., Chen Q., Xie Y., Ying S., Chen Z., Xiao B., Hu Y. Decreased expression of hsa_circ_0003570 in hepatocellular carcinoma and its clinical significance. J. Clin. Lab. Anal. 2018;32:e22239. doi: 10.1002/jcla.22239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim G., Han J.R., Park S.Y., Tak W.Y., Kweon Y.O., Lee Y.R., Han Y.S., Park J.G., Kang M.K., Lee H.W., et al. Circular noncoding RNA hsa_circ_0005986 as a prognostic biomarker for hepatocellular carcinoma. Sci. Rep. 2021;11:14930. doi: 10.1038/s41598-021-94074-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeck W.R., Sharpless N.E. Detecting and characterizing circular RNAs. Nat. Biotechnol. 2014;32:453–461. doi: 10.1038/nbt.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeck W.R., Sorrentino J.A., Wang K., Slevin M.K., Burd C.E., Liu J., Marzluff W.F., Sharpless N.E. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA. 2013;19:141–157. doi: 10.1261/rna.035667.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown J.R., Chinnaiyan A.M. The Potential of Circular RNAs as Cancer Biomarkers. Cancer Epidemiol. Biomark. Prev. 2020;29:2541–2555. doi: 10.1158/1055-9965.EPI-20-0796. [DOI] [PubMed] [Google Scholar]
- 17.Zhang P.F., Wei C.Y., Huang X.Y., Peng R., Yang X., Lu J.C., Zhang C., Gao C., Cai J.B., Gao P.T., et al. Circular RNA circTRIM33-12 acts as the sponge of MicroRNA-191 to suppress hepatocellular carcinoma progression. Mol. Cancer. 2019;18:105. doi: 10.1186/s12943-019-1031-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu J., Xu Q.G., Wang Z.G., Yang Y., Zhang L., Ma J.Z., Sun S.H., Yang F., Zhou W.P. Circular RNA cSMARCA5 inhibits growth and metastasis in hepatocellular carcinoma. J. Hepatol. 2018;68:1214–1227. doi: 10.1016/j.jhep.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 19.Qiu L., Huang Y., Li Z., Dong X., Chen G., Xu H., Zeng Y., Cai Z., Liu X., Liu J. Circular RNA profiling identifies circADAMTS13 as a miR-484 sponge which suppresses cell proliferation in hepatocellular carcinoma. Mol. Oncol. 2019;13:441–455. doi: 10.1002/1878-0261.12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.