Abstract
Bacterial prostatitis infections are described as infections that are difficult-to-treat, due to prostate anatomic characteristics along with clinical difficulty in terms of diagnosis and management. Furthermore, the emergence of multidrug resistant (MDR) bacteria, such as extended-spectrum beta-lactamase (ESBL) producer Escherichia coli, also representing the main causative pathogen in prostatitis, poses major problems in terms of antibiotic management and favorable clinical outcome. Oral fosfomycin, an antibiotic commonly used for the treatment of uncomplicated urinary tract infections (UTIs), has been recently evaluated for the treatment of bacterial prostatitis due to its favorable pharmacokinetic profile, its activity against MDR gram-positive and gram-negative bacteria, safety profile, and multiple synergic effect with other antibiotics as well as the low resistance rate. This review addresses fosfomycin pharmacokinetics and pharmacodynamics and discusses the latest clinical evidence on its clinical use to treat acute and chronic bacterial prostatitis in hospitalized patients and in outpatients. As described in several reports, oral fosfomycin may represent a valid therapeutic option to treat susceptible germs commonly causing prostatitis, such as E. coli and other Enterobacterales as well as Enterococcus faecium, even as a first-line regimen in particular clinical settings (patients with previous treatment failure, with allergies or outpatients). Stronger data from further studies, including randomized controlled trials, would be helpful to establish the proper dosage and specific indications.
Keywords: oral fosfomycin, bacterial prostatitis, urinary tract infections
1. Introduction
Male urinary tract infections (UTI) have an overall estimated prevalence between 1.5% and 9%, worldwide [1]. Among them, acute (ABP) and chronic bacterial prostatitis (CBP)—according to the National Institutes of Health (NIH), the classification is also known as category I prostatitis (CIP) and category II prostatitis (CIIP), respectively [2]—are considered cumbersome-to-treat infections owing to limited antibiotic choices and poor drug distribution in prostatic tissue [3]. Escherichia coli is considered the main causative agent of both ABP and CBP, although other organisms, including Enterococcus spp., Klebsiella spp., and Proteus spp., are also rising [1,3,4,5,6,7]. Furthermore, the increased prevalence of challenging antibiotic resistant microorganisms, such as extended spectrum beta-lactamases (ESBL) producing E. coli, as well as the increasing fluoroquinolone resistance, pose major clinical problems in choosing the appropriate therapy to treat and eradicate UTIs [3,8]. In fact, ESBLs are beta-lactamases able to provide bacterial resistance by hydrolyzing various antibiotics: penicillins, first-, second-, and third generation cephalosporins, as well as aztreonam [9]. For this reason, infections caused by ESBL producer bacteria require the administration of different antibiotic classes, such as fluoroquinolones—that may produce adverse effects due to their known toxicities—or carbapenems, which should be considered last resort drugs that should be spared and can be used only in hospital settings [7] [10]. Alarmingly, the increasing rate of Enterobacterales carbapenem resistance (mainly due to carbapenemases’ production), adds additional complications to the treatment of both ABP and CPB [4,8]. An old and well-known ally for the treatment of UTIs sustained by these bacteria is represented by fosfomycin. Since its approval, oral fosfomycin–trometamol formulation has been used to treat uncomplicated cystitis in women provoked by susceptible micro-organisms, due to both fosfomycin’s favorable capacity to gain high bladder concentrations, even after single dose, and to its capacity to not allow bacteria to develop cross-resistance [11,12]. This review briefly reports fosfomycin’s history, pharmacokinetic and pharmacodynamic properties and then the analyses of the data available from scientific literature about fosfomycin–trometamol use in patients with acute and chronic prostatitis. Thanks to its advantageous safety along with its ability to achieve therapeutic concentrations in prostatic secretions, fosfomycin–trometamol results in a valid therapeutic option for the treatment of both ABP and CBP, especially in some settings [3].
2. Fosfomycin Pharmacology
Fosfomycin was discovered in 1969 by Hendlin et al. under the name of phosphonomycin. It is a phosphoenolpyruvate analogue isolated in fermentation broths through experimentation with various strains of Streptomyces spp., such as, S. fradiae, S. viridochromogenes, and S. wedmorensis [13].
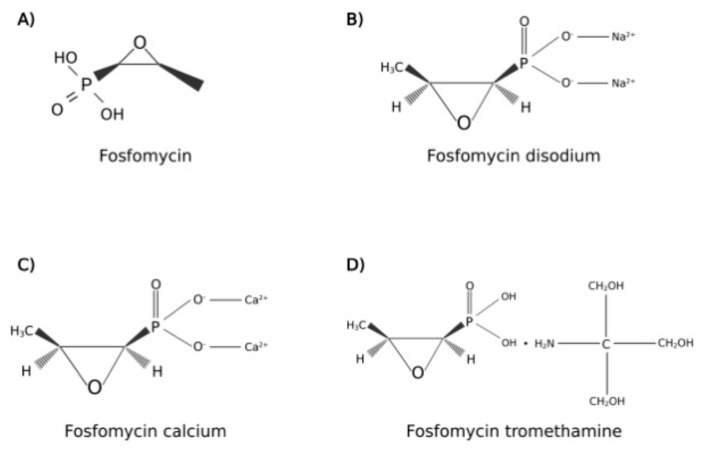
The molecular structure of fosfomycin (Figure 1) differs in consideration of drug formulation, indeed, it is available in two oral formulations, fosfomycin calcium and fosfomycin trometamol (also known as fosfomycin tromethamine); this latter is a soluble salt with better bioavailability than fosfomycin calcium. Moreover, an intravenous formulation, fosfomycin disodium, is also commercialized for diverse clinical uses, different from the scope of this review [14].
Figure 1.
Molecular structures of (A) fosfomycin; (B) fosfomycin disodium; (C) fosfomycin calcium; (D) fosfomycin tromethamine. Created with BioRender.com; accessed on 2 August 2022.
2.1. Mechanism of Action
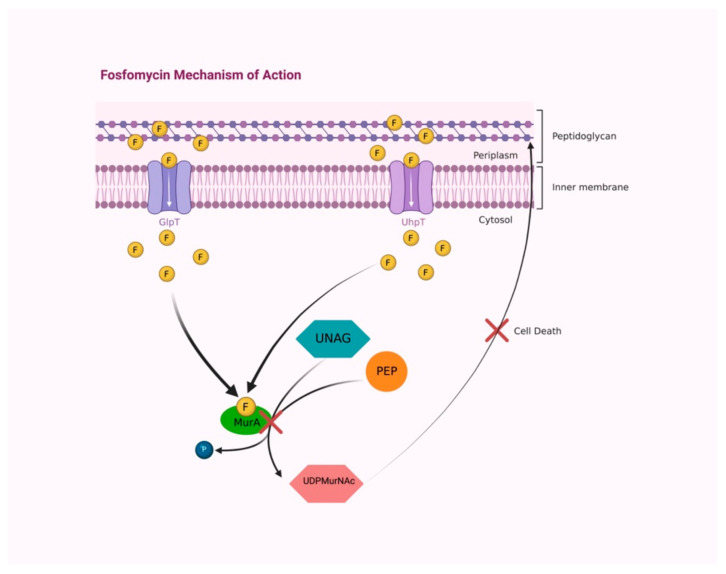
Fosfomycin is a concentration-dependent bactericidal agent with broad bactericidal activity against both gram-positive and gram-negative bacteria, carried out by interfering with the formation of the peptidoglycan precursor UDP N-acetylmuramic acid (UDPMurNAc), the first cytoplasmic step of bacterial cell wall biosynthesis. The enzyme UDP-N-acetylglucosamine enolpyruvyl transferase (MurA) is involved in peptidoglycan biosynthesis by catalyzing the transfer of the enolpyruvyl moiety of phosphoenolpyruvate to the 3′-hydroxyl group of UDP-N-acetylglucosamine (UNAG). Fosfomycin covalently binds the thiol group of a cysteine in the active site of MurA, inactivating it (Figure 2) [15,16].
Figure 2.
Fosfomycin accesses into the bacterial wall by two transport uptake systems, GlpT and UhpT. In the cytoplasm, fosfomycin covalently binds to the active site of MurA enzyme, preventing the reaction between PEP and UNAG and avoiding UDPMurNAc synthesis, resulting in peptidoglycan building interruption and causing bacterial-cell death. Abbreviations: F, fosfomycin; GlpT, L-alpha- glycerophosphate transport system; UhpT, hexose-6-phosphate transport system; PEP, phosphoenolpyruvate; UNAG, UDP-N-acetylglucosamine; MurA, UDP-N-acetylglucosamine enolpyruvyl transferase; P, phosphate; UDPMurNAc, UDP N-acetylmuramic acid. Created with BioRender.com; accessed on 3 August 2022.
Two transport uptake systems provide drug access into the bacterial cell, the constitutively functional L-alpha-glycerophosphate transport system (GlpT), induced by glyceraldehyde-3-phosphate, and the hexose-6-phosphate transport systems (UhpT), induced by an extracellular hexose monophosphate inductor, glucose-6-phosphate (G6P) [17]. Its unique mechanism of action-targeting early steps of cell wall synthesis without negatively interfering with other molecules-plays a key role in synergism with other antibiotics, including beta-lactams, aminoglycosides, and fluoroquinolones [14].
2.2. Antimicrobial Spectrum
Fosfomycin has broad-spectrum bactericidal activity against staphylococci, enterococci, Haemophilus spp., and most enteric gram-negative bacteria. It also has excellent activity against most E. coli, including 95.5% of ESBL producing E. coli [18]. Conversely, ESBL 025b/B2 E. coli strains are resistant to fosfomycin. [18] Klebsiella spp. and Serratia spp. have higher MICs (from 0.25 to 512 µg/mL for Klebsiella spp. with an ECOFF of 128 µg/mL and from 0.5 to 128 µg/mL for Serratia spp. with an ECOFF of 32 µg/mL); fosfomycin has activity against only 57.6% of ESBL-producing Klebsiella spp [19]. Pseudomonas aeruginosa is variably susceptible to fosfomycin, with MICs ranging from 4 to more than 512 µg/mL and with an ECOFF of 256 µg/mL [18].
Nearly all isolates of Acinetobacter baumannii are resistant to fosfomycin; however, several studies provided evidence of a fosfomycin synergistic effect with amikacin, colistin, and sulbactam to treat Acinetobacter infections [20]. Fosfomycin retains excellent in vitro activity against both E. faecalis (97.7%) and E. faecium (100%) [21]. Moreover, a significant intracellular bactericidal effect was observed for fosfomycin in osteoblast cells infected by Staphylococcus aureus [22]. Due to its unique mechanism of action, cross-resistance with other antibiotics is uncommon [23].
2.3. Resistance Mechanisms
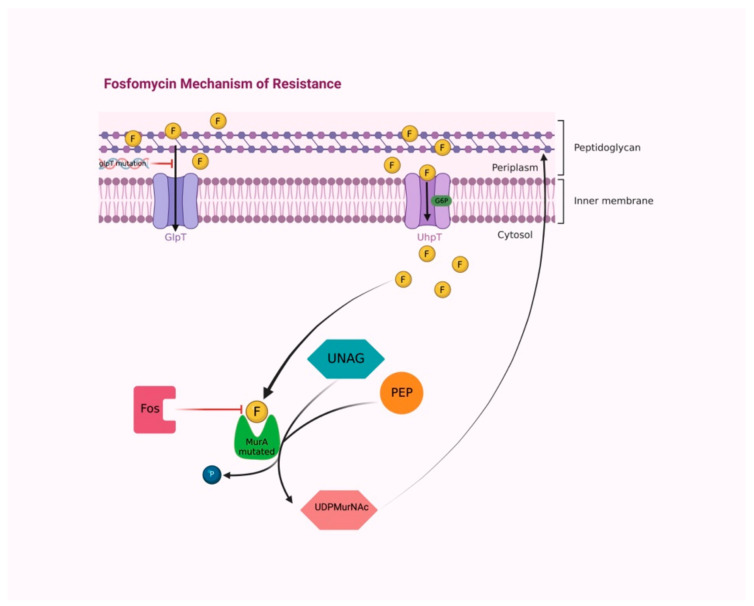
Mechanisms that confer bacterial resistance to fosfomycin are chromosomally or plasmid-mediated [24]. Most resistance is chromosomally mediated and interferes with the transport of the antibiotic into the bacteria [25]. Concerning the chromosomal one, mutants defective in either the primary entry system via GlpT or in an alternative transport mechanism mediated by UhpT have been demonstrated to reduce fosfomycin intracellular uptake [26]. Nevertheless, the existence of a functional G6P-inducible UhpT transport system often overrules resistance related to mutations in GlpT, resulting in a fosfomycin susceptible phenotype (Figure 3) [27].
Figure 3.
The majority of fosfomycin resistance mechanisms are chromosomally mediated, interfering with the antibiotic transport into bacteria. Mutations of the glpT transporter gene cause reduced fosfomycin permeability. The functional G6P-inducible UhpT transport system overrules resistance, maintaining fosfomycin permeability. Cysteine/aspartate substitution in the active site of MurA provokes conformational modification that prevents fosfomycin binding. Fos enzymes inactivate fosfomycin by modifying its molecular structure. Abbreviations: F, fosfomycin; GlpT, L-alpha-glycerophosphate transport system; UhpT, hexose-6-phosphate transport system; G6P, glucose-6-phosphate; PEP, phosphoenolpyruvate; UNAG, UDP-N-acetylglucosamine; MurA, UDP-N-acetylglucosamine enolpyruvyl transferase; P, phosphate; UDPMurNAc, UDP N-acetylmuramic acid; Fos, fos enzymes. Created with BioRender.com; accessed on 4 August 2022.
Experiments on fosfomycin-resistant strains of E. coli allowed for the identification of defects in phosphoenolpyruvate—sugar phosphotransferase system (PTS) and adenylate cyclase activity, resulting in cAMP level reduction and a minor induction of the GlpT transport system [28]. Low levels of cAMP can also occur because of mutations in the cyaA gene that codes for adenylate cyclase [29].
Alternatively, fosfomycin may be inactivated by mutations, which allow pyruvyl transferase to distinguish between the substrate phosphoenolpyruvic acid and fosfomycin [30]. Plasmid-mediated fosA, fosB, fosC, fosX are metalloenzymes belonging to the glyoxalase superfamily and inactivate fosfomycin by catalyzing its conjugation with glutathione or another nucleophile [31].
Speaking generally, gram-negative (P. aeruginosa) bacteria express FosA, a Mn2+ and K+-dependent glutathione transferase; whilst gram-positive bacteria (Listeria monocytogenes and S. aureus) produce FosB and FosX, which are a Mg2+-dependent L-cysteine thiol transferase and Mn2+-dependent fosfomycin-specific epoxide hydrolase, respectively [29,32].
FosC utilizes ATP and exerts its activity by adding to fosfomycin a phosphate group, which also nullifies its antimicrobial properties [33]. These enzymes act by nucleophilic attack on the carbon 1 of fosfomycin, which opens the epoxide ring and inactivates it [34].
Regarding the mechanisms of intrinsic resistance, the lack of fosfomycin effectiveness could result also from the modification of the drug’s target, MurA. Indeed, the substitution of cysteine with aspartate in its active site is established to prevent fosfomycin binding to MurA [35].
2.4. Pharmacokinetic Properties
Following oral administration, fosfomycin is rapidly absorbed in the small intestine, where the bioavailability ranges between 34 and 58% for fosfomycin trometamol, whereas it is around 12% for fosfomycin calcium [14]. Apparently, the absorption is not influenced by individual age, although its rate and extent seem to be reduced by food intake (37% fasting versus 30% with food). Additionally, the serum maximum concentration (Cmax) is also higher under fasting conditions, but urinary recovery rates are similar (58% versus 52%) [17].
A pharmacokinetic comparative study, performed in 1988 by Borsa and colleagues in young and elderly adults, demonstrated that fosfomycin trometamol has a higher bioavailability compared to calcium salt. Moreover, at a dose of 2–3 g, the mean peak serum of tromethamine salt was founded to be 2-to-4-fold greater than the one produced by fosfomycin calcium [36].
Those findings probably depend on hydrolyzation and consequently, the inactivation of calcium by gastric acids [37,38,39]. Furthermore, in terms of rate and extent of absorption, a difference between the two oral formulations can be noticed. Two hours after drug administering, the uptake of fosfomycin trometamol was resulted to be six times higher than that of fosfomycin calcium, whereas twelve hours later it was approximately 4 times higher. The binding between antibiotic and plasma proteins is marginal [39].
Following oral administration of fosfomycin trometamol, the mean apparent steady-state volume of distribution (Vss) is 136.1 (±44.1) L. Fosfomycin is not bound to plasma proteins. Oral fosfomycin is distributed to the kidneys, bladder wall, prostate, and seminal vesicles [40].
From 30 to 60% of fosfomycin trometamol is excreted unmodified in the urine vs. 9–18% for the calcium salt. The mean serum elimination half-life (t1/2) of fosfomycin trometamol is estimated at 5.7 h. The area under the concentration time curve (AUC) is 145 to 228 mg h/L [41]. The elimination happens for about 95% through glomerular filtration in the kidneys and no tubular secretion phenomena occur [14]. In addition, oral fosfomycin formulations are subjected to enterohepatic recirculation. Peak urinary concentrations were reported to reach 4000 mg/L and to remain at concentrations >100 mg/L for 48 h [42].
2.5. Adverse Drug Reaction
Guidelines about oral fosfomycin administration for uncomplicated UTIs (mostly in women) establish short regimens (single/two 3 g doses), which are unlikely to be responsible for major side effects. Furthermore, oral fosfomycin results to be a well-tolerated drug with only mild gastrointestinal side effects (mainly diarrhea), reported either with a fosfomycin dosage higher than 3 g daily or for prolonged treatment [3].
Nonetheless, in clinical trials, the most frequently described adverse events arising in > 1% of the study population are: diarrhea—10.4%, headache—10.3%, vaginitis—7.6%, nausea—5.2%, rhinitis—4.5%, back pain—3.0%, dysmenorrheal—2.6%, pharyngitis— 2.5%, dizziness—2.3%, abdominal pain—2.2%, pain—2.2%, dyspepsia—1.8%, asthenia —1.7%, and rash —1.4%. Other side events developed by patients following fosfomycin administration, with a rate of less than 1% are: abnormal stools, anorexia, constipation, dry mouth, dysuria, ear disorder, fever, flatulence, flu syndrome, hematuria, infection, insomnia, lymphadenopathy, menstrual disorder, migraine, myalgia, nervousness, paresthesia, pruritus, SGPT increased, skin disorder, somnolence, and vomiting [43].
3. Rationale for Oral Fosfomycin Administration in Patients with Bacterial Prostatitis
Among antibiotics in clinical use, fosfomycin tromethamine, with a low molecular weight of 138.059 + 121.131 g/mol, is able to reach clinically relevant concentrations in the bladder as well as in the prostatic gland. Its hydrophilicity, together with the negligible protein binding (<5%) and the overall PK/PD profile, allow the antibiotic to reach a bio-availability level of 33–50% with a 2-h concentration of 20–30 mg/L and 2000–2500 mg/L in the serum and in the urine, respectively, after a single oral dose of 3 g [44].
Following oral fosfomycin administration in animal models, Fan et al. described the beneficial effects in terms of decreased inflammation, lowering bacterial proliferation and the amelioration of prostatic damage [45].
To note, as reported by the European Committee on Antimicrobial Susceptibility Testing (EUCAST), fosfomycin epidemiological cut-offs (ECOFF) for the bacteria most frequently isolated in UTIs range from 4 mg/L for E. coli to 8 mg/L for Proteus mirabilis and 32 mg/L for S. aureus (even lower for MRSA) [46,47], values that are lower than the antibiotic concentration in the urine even after 48 h post antibiotic administration (100–700 mg/L) [44]. Several studies do not endorse fosfomycin administration if its MIC is >4 mg/L, due to the high risk of not achieving efficient intraprostatic concentrations [1].
The reason behind its success in the treatment of UTIs as well as the perioperative prophylaxis of prostate biopsy is due to its unchanged excretion in the urine and almost unchanged renal elimination [44]. On the other hand, this poses a problem in the administration of fosfomycin to patients with compromised renal function.
Although oral fosfomycin administration dates back to the 1970s and some mechanisms of resistances are unknown—as aforementioned—fosfomycin-resistant E. coli are still rare, testifying the slow adaptation rate of the bacteria to this molecule, a clear advantage for the physician. Moreover, the mechanism of action of fosfomycin, affecting the early steps involved in bacterial cell wall formation [48], suggests an additive or synergistic action in combination with other antibiotics; in fact, fosfomycin shows important synergistic effects with many other antibiotics, e.g., piperacillin/tazobactam, ceftazidime/avibactam, meropenem, colistin, and daptomycin, as well as linezolid [14,44,49,50,51,52,53,54,55,56,57,58,59].
Some issues can arise in performing fosfomycin susceptibility testing due to the aforementioned fosfomycin mechanism of entry requiring the presence of G6P. Because of that, the gold standard method for Staphylococci, Enterococci, Enterobacterales and P. aeruginosa is the agar dilution with the addition of G6P in the medium [60]. This methodology is time and consumables demanding, which is not suitable for every hospital setting. Luckily, more easy and rapid antimicrobial susceptibility testing methods have been produced by different companies and several studies have been performed demonstrating the validity of the disk diffusion and gradient test, as well as automatized methods [61,62,63,64,65,66]. The accuracy of the results depends on the choice of the most appropriate method for the isolate species, and on the strict adherence to the manufacturers’ instructions, as reported by EUCAST [67].
Last but not least, when performing the disk diffusion or gradient test, it could be possible to see colonies within the inhibition zone. In accordance with the EUCAST recommendations, these colonies must be ignored. Luckily, to date, there is no correlation between their appearance and the onset of fosfomycin resistance, which remains very low [68,69].
4. Oral Fosfomycin in Chronic Bacterial Prostatitis
Bacterial prostatitis infections, both acute and chronic, are challenging to treat infections, due to the poor antibiotic penetration in prostatic tissue. CBP represents a complex setting to deal with, due to the presence of prostatic calcifications acting as a bacterial sanctuary, and the bacterial biofilm formation, which both lead to relapsing infections, persisting symptoms, and treatment failure; because of this, antibiotic treatment for CBP requires longer duration compared to acute forms, resulting in increased resistance due to selective pressure [3].
Bouiller et al. retrospectively described 17 CBP episodes treated with oral fosfomycin, 3 g every 24–48 h, for a mean duration of 5.5 weeks, achieving a microbiological and clinical cure in more than 90% of episodes. Oral fosfomycin was prescribed mostly to treat CBP due to ESBL producing Enterobacterales with resistance to fluoroquinolones and cotrimoxazole in patients with underlying urological disorders, which could explain the incidence of recurrences (58%) in that population [1] (Table 1).
Table 1.
Clinical use of oral fosfomycin in acute and chronic bacterial prostatitis, causative strains, dose regimens, side effects, and outcomes.
| Prostatitis Type | Pathogen (n° of Isolates) | Combination Therapy | Fosfomycin Dosage | Adverse Effect | Clinical Cure | Microbiological Cure | Reference |
|---|---|---|---|---|---|---|---|
| CBP | E. coli (12) | 3/12 | 3 g/24–48 h for 5.5 weeks (mean duration) | Diarrhea (4/12) | Yes | 8/12 | [1] |
| K. pneumoniae (5) | 1/5 | - | 4/5 | 3/5 | |||
| E. coli (14) | 1/14 | 3 g/48–72 h for 6 weeks | - | 7/14 | 8/14 | [70] | |
| K. oxytoca | No | No | No | ||||
| E. coli (29) | No | 3 g/24 h for the first week, then 3 g/48 h or 3 g/72 h for 6–13 weeks | Diarrhea (4/44) | 23/29 | 23/29 | [71] | |
| K. oxytoca (3) | 3/3 | 3/3 | |||||
| K. pneumoniae (3) | 2/3 | 2/3 | |||||
| P. mirabilis (2) | 2/2 | 1/2 | |||||
| P. aeruginosa | No | No | |||||
| E. faecalis (6) | 5/6 | 6/6 | |||||
| ESBL-E. coli | No | 3 g/24 h for 9 days, then 3 g/48 h for 3 months and 3 g/weekly for 9 months | Diarrhea during the first week | Yes | Yes | [72] | |
| ESBL-E. coli | No | 15 weeks of 3 g once daily; 5 days of 3 g twice daily | Diarrhea with the doubled dose | Yes | Yes | [73] | |
| ESBL-E. coli | Yes | 3 g/72 h | - | Yes | Yes | [74] | |
| E. coli | No | 3 g/24 h for 1 week, then 3 g/48 h for 3 months | - | Yes | Yes | [75] | |
| R. planticola | No | 3 g/48 h for 3 months | - | Yes | Yes | [76] | |
| ABP | ESBL-E. coli | No | 3 g/24 h for 1 week—3 g/48 h for 2 weeks | Diarrhea during the first week | Yes | Yes | [77] |
| ESBL-E. coli | Yes | 3 g/24 h—3 g/twice daily (5 days) for 16 weeks | Diarrhea with the doubled dose | Yes | Yes | [73] | |
| E. faecium | No | 3 g/72 h for 3 weeks | - | Yes | Yes | [78] |
Los-Arcos et al. reported 15 complicated cases of CBP, of which 14 were caused by E. coli (4 isolates produced ESBL), whilst in one case the identified etiological agent was K. oxytoca; 13 patients received 3 g every 72 h of oral fosfomycin and 2 patients received 3 g every 48 h. Overall, treatment duration was 6 weeks, attaining microbiological examination in 8 of 15 cases, whereas a clinical cure was achieved in 7 of 15 cases; 7 cases failed the fosfomycin therapy within a median follow-up period of 29 months; four out of the six patients diagnosed with prostatic calcifications relapsed within six months [70].
Karaiskos et al. described 44 CBP cases treated with oral fosfomycin, 38 of which had a positive culture for gram-negative bacteria (mostly E. coli): 10 isolates produced ESBL, 26 cases were MDR, whilst 6 cultures tested positive for E. faecalis. All patients received 3 g daily of oral fosfomycin for one week, followed by 3 g every 48 h for 6 weeks and 12 weeks, in 25 patients and in 19 patients, respectively. A microbiological cure was achieved in 86% of the cases at the end of therapy and 77% at 6 months; the clinical cure was 84% at the end of therapy and 80% at 6 months. Fosfomycin failure was observed in 18% of patients, the majority of whom had a MIC > 16 mg/L [71].
Almeida et al. described a case of E. coli CBP with several relapsing episodes, despite multiple previous antibiotic regimens; the patient was treated with prolonged fosfomycin therapy: 3 g daily for 10 days, then 3 g every 48 h for three months followed by 3 g weekly for 9 months. Due to intraprostatic calcifications, besides fosfomycin therapy, the patient underwent transurethral resection of the prostate (TURP), achieving a clinical cure confirmed after 9 months of post-therapy follow-up [72].
In a case report, Grayson et al., trying to enhance the antibacterial activity towards an ESBL-E. coli responsible for a CBP case, which relapsed after carbapenem therapy, administered 3 g twice daily of oral fosfomycin, causing intense diarrhea and leading clinicians to reduce the dosage at 3 g daily. A microbiological and clinical cure was achieved 6 months after the end of therapy [73].
Cunha et al. described the case of an ESBL E. coli prostatitis in a patient with a penicillin allergy and affected by prostatic hypertrophy; he did not respond to previous therapies with nitrofurantoin, doxycycline, and oral fosfomycin, showing persisting pyuria and bacteriuria as well as persistent positive urine cultures. A prostatic ultrasound revealed intraprostatic calcifications, without abscesses, and a TURP was performed to remove them.
Therefore, a three-week regimen of 3 g daily of oral fosfomycin plus doxycycline 100 mg twice daily was made, fulfilling a sustained microbiological and clinical cure. Of note, the E. coli strains isolated resulted in always being susceptible to fosfomycin (MIC < 4 μg/L) [74].
Recently, Denes described a clinical case about a patient, with a medical history of prostatic surgery and urethral stenosis, who was treated with a course of oral fosfomycin (3 g daily for one week followed by 3 g every 48 h for 3 months) to treat a prostatitis due to E. coli resistant to fluroquinolones and cotrimoxazole, and achieved a microbiological and clinical cure in the six-month follow-up. Just as in the case of Cunha et al., previous fosfomycin administration did not result in bacteria resistance [75].
Finally, an uncommon case of persistent CBP, in a patient affected by prostatic hypertrophy and ciprofloxacin allergy due to Raoultella planticola, was successfully treated by Gian et al. who administer oral fosfomycin, 3 g daily, for three months [76].
The majority of reported CBP patients received oral fosfomycin as an alternative regimen to the standard of care without standardized duration and dosage, achieving microbiological/clinical eradication and a recurrence rate not so different compared with the classic agent (fluoroquinolones). Prostatic calcifications, along with urinary abnormalities, represent the main causes, which led to antibiotic failure and infection recurrence. Oral fosfomycin did not demonstrate a safety concern, except for self-limiting diarrhea, and previous fosfomycin treatments have not been associated with resistance development [1,70,71,72,73,74,75,76].
5. Oral Fosfomycin in Acute Bacterial Prostatitis
Due to prostate inflammatory status in ABP, most antibiotics, including fosfomycin, do penetrate the prostatic tissue [79]. Although the pharmacokinetic and pharmacodynamic proprieties of fosfomycin are very favorable and fosfomycin pharmacological proprieties—especially its low protein binding and high lipid solubility [2], which promote its penetration into the lipid-rich prostatic parenchyma—the use of fosfomycin for the treatment of ABP is still neglected.
Even though oral administration of fosfomycin trometamol does not represent the first choice for the treatment of ABP, it could be useful for outpatients or for those who cannot receive other drugs due to allergies, as well as being an adjuvant of other antibiotics due to its good synergistic effect and the low rate of resistance [12].
Despite the use of oral fosfomycin for the treatment of urinary tract infections and, somewhat, for the management of CBP, which is common, only a few cases reported the efficacy of oral fosfomycin in the treatment of ABP sustained by diverse bacterial species: in two cases the microorganism responsible for the ABP and treated with fosfomycin was an ESBL producing E. coli [73,77], whilst E. faecium [78] has been reported only once.
With regards to ESBL E. coli cases, of note is the age difference between the two patients, the 30-year-old [77] and 73-year-old [73]. The younger patient was treated with oral fosfomycin for three weeks under a dose regimen of 3 g once daily for the first week, then switched to 3 g once every 48 h for the remaining two weeks [77]. The older patient received 3 g once daily of oral fosfomycin for 16 weeks [73]. In both cases, the regimen was set in response to the development of diarrhea when the clinicians tried to use a higher dose regimen of antibiotic. The pathogen eradication was reached in both the cases, revealing that the efficacy of fosfomycin seems to not be strictly related to the dosage due to its high concentration in the inflamed prostate.
The only ABP caused by a gram-positive bacterium and treated with fosfomycin reported in literature was sustained by an E. faecium resistant to ampicillin, chloramphenicol, vancomycin, gentamycin, and ciprofloxacin, but susceptible to nitrofurantoin and quinupristin-dalfopristin. To note, the strain had a high fosfomycin MIC of 64 mg/L, considered as an intermediate susceptibility by the authors, which nowadays represents the cut-off of susceptibility for E. faecalis according to the clinical and laboratory standards institute (CLSI) [80]. Due to the 85-year-old patient’s refusal of intra-venous (IV) therapy and due to the scarce penetration of nitrofurantoin into the prostate, he was treated with a trial of prolonged and unconventional dosing of oral fosfomycin equal to 3 g every 3 days for 21 days. The patient’s symptoms resolved after the second dose and the follow-up of two years demonstrated no recurrence of the infection [78].
According to other authors, fosfomycin could be considered as an alternative therapy for quinolone-resistant ABP [81] or in combination with cefoxitin in its IV form for the treatment of ABP caused by fosfomycin susceptible Enterobacterales, paying attention to the heart and renal functionality [82].
6. Conclusions
Bacterial prostatitis infections have always represented difficult-to-treat infections because of the challenging diagnosis, and it is often laborious due to unclear clinical signs and prostate anatomical characteristics, which make it a cumbersome target to reach for antibiotics and leads to the concept of the infection “sanctuary”. Furthermore, the emergence of MDR microorganisms poses additional therapeutical problems, especially for frail patients, such as the elderly and immunocompromised [83,84,85,86].
Several studies demonstrated that oral fosfomycin possesses an interesting pharmacokinetic and pharmacodynamic profile, which allows greater intraprostatic drug concentrations, which are frequently sufficient to achieve bactericidal effects. However, testing methods and MIC evaluation should be always cautiously observed.
The evidence we reported is more representative for CBP than ABP, and mainly regards E. coli infections. Fosfomycin has been used in prolonged course regimen, for both ABP and CBP, and its use was often limited to MDR bacteria, previous treatment failure, and intolerance to previous treatments.
Despite the lack of randomized controlled trials and stronger clinical data, which may be a determining factor, fosfomycin could represent a valid therapeutic option to treat prostatitis caused by susceptible germs, perhaps as first-line regimen in particular clinical settings.
Abbreviations
| ABP | Acute bacterial prostatitis |
| ATP | Adenosine triphosphate |
| AUC | Area under the concentration time curve |
| CIP | Category I prostatitis |
| CIIP | Category II prostatitis |
| CBP | Chronic bacterial prostatitis |
| CLSI | Clinical and laboratory standards institute |
| cAMP | Cyclic adenosine monophosphate |
| ECOFF | Epidemiological cut-off |
| EUCAST | European Committee on Antimicrobial Susceptibility Testing |
| ESBL | Extended spectrum beta-lactamases |
| G6P | Glucose-6-phosphate |
| UhpT | Hexose-6-phosphate transport systems |
| IV | Intra-venous |
| GlpT | L-alpha-glycerophosphate transport system |
| MRSA | Methicillin resistant Staphylococcus aureus |
| MIC | Minimum inhibitory concentration |
| MDR | Multidrug resistant |
| NIH | National Institutes of Health |
| PD | Pharmacodynamic |
| PK | Pharmacokinetic |
| PTS | Sugar phosphotransferase system |
| TURP | Transurethral resection of the prostate |
| UDPMurNAc | UDP N-acetylmuramic acid |
| UNAG | UDP-N-acetylglucosamine |
| UTI | Urinary tract infections |
| Vd | Volume of distribution |
Author Contributions
Conceptualization, A.M. and S.S.; writing—original draft preparation, C.M.B., E.A. and M.C.; writing—review and editing, G.N., G.C. and R.B.; supervision, B.C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bouiller K., Zayet S., Lalloz P.E., Potron A., Gendrin V., Chirouze C., Klopfenstein T. Efficacy and Safety of Oral Fosfomycin-Trometamol in Male Urinary Tract Infections with Multidrug-Resistant Enterobacterales. Antibiotics. 2022;11:198. doi: 10.3390/antibiotics11020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.NIH Consensus Definition and Classification of Prostatitis|JAMA|JAMA Network. [(accessed on 4 July 2022)]. Available online: https://jamanetwork.com/journals/jama/article-abstract/1030245.
- 3.Kwan A.C.F., Beahm N.P. Fosfomycin for bacterial prostatitis: A review. Int. J. Antimicrob. Agents. 2020;56:106106. doi: 10.1016/j.ijantimicag.2020.106106. [DOI] [PubMed] [Google Scholar]
- 4.Erdem H., Hargreaves S., Ankarali H., Caskurlu H., Ceviker S.A., Bahar-Kacmaz A., Meric-Koc M., Altindis M., Yildiz-Kirazaldi Y., Kizilates F., et al. Managing adult patients with infectious diseases in emergency departments: International ID-IRI study. J. Chemother. 2021;33:302–318. doi: 10.1080/1120009X.2020.1863696. [DOI] [PubMed] [Google Scholar]
- 5.El-Sokkary R., Uysal S., Erdem H., Kullar R., Pekok A.U., Amer F., Grgić S., Carevic B., El-Kholy A., Liskova A., et al. Profiles of multidrug-resistant organisms among patients with bacteremia in intensive care units: An international ID-IRI survey. Eur. J. Clin. Microbiol. Infect. Dis. 2021;40:2323–2334. doi: 10.1007/s10096-021-04288-1. [DOI] [PubMed] [Google Scholar]
- 6.Marino A., Munafò A., Zagami A., Ceccarelli M., Di Mauro R., Cantarella G., Bernardini R., Nunnari G., Cacopardo B. Ampicillin plus ceftriaxone regimen against enterococcus faecalis endocarditis: A literature review. J. Clin. Med. 2021;10:4594. doi: 10.3390/jcm10194594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gajdács M., Ábrók M., Lázár A., Burián K. Epidemiology and antibiotic resistance profile of bacterial uropathogens in male patients: A 10-year retrospective study. Farmacia. 2021;69:530–539. doi: 10.31925/farmacia.2021.3.16. [DOI] [Google Scholar]
- 8.Magiorakos A.P., Srinivasan A., Carey R.B., Carmeli Y., Falagas M.E., Giske C.G., Harbarth S., Hindler J.F., Kahlmeter G., Olsson-Liljequist B., et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 9.Paterson D.L., Bonomo R.A. Extended-spectrum β-lactamases: A clinical update. Clin. Microbiol. Rev. 2005;18:657–686. doi: 10.1128/CMR.18.4.657-686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaviria L.P., Montsant L., Azuaje C., González-Díaz A., Horcajada J.P., Limón E., Viñas M., Espinal P., Fusté E. A Descriptive Analysis of Urinary ESBL-Producing-Escherichia coli in Cerdanya Hospital. Microorganisms. 2022;10:488. doi: 10.3390/microorganisms10030488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aris P., Boroumand M.A., Rahbar M., Douraghi M. The Activity of Fosfomycin Against Extended-Spectrum Beta-Lactamase-Producing Isolates of Enterobacteriaceae Recovered from Urinary Tract Infections: A Single-Center Study over a Period of 12 Years. Microb. Drug Resist. 2018;24:607–612. doi: 10.1089/mdr.2017.0097. [DOI] [PubMed] [Google Scholar]
- 12.Falagas M.E., Kastoris A.C., Kapaskelis A.M., Karageorgopoulos D.E. Fosfomycin for the treatment of multidrug-resistant, including extended-spectrum β-lactamase producing, Enterobacteriaceae infections: A systematic review. Lancet Infect. Dis. 2010;10:43–50. doi: 10.1016/S1473-3099(09)70325-1. [DOI] [PubMed] [Google Scholar]
- 13.Hendlin D., Stapley E.O., Jackson M., Wallick H., Miller A.K., Wolf F.J., Miller T.W., Chaiet L., Kahan F.M., Foltz E.L., et al. Phosphonomycin, a new antibiotic produced by strains of streptomyces. Science. 1969;166:122–123. doi: 10.1126/science.166.3901.122. [DOI] [PubMed] [Google Scholar]
- 14.Michalopoulos A.S., Livaditis I.G., Gougoutas V. The revival of fosfomycin. Int. J. Infect. Dis. 2011;15:e732–e739. doi: 10.1016/j.ijid.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Eschenburg S., Priestman M., Schönbrunn E. Evidence that the fosfomycin target Cys115 in UDP-N-acetylglucosamine Enolpyruvyl Transferase (MurA) is essential for product release. J. Biol. Chem. 2005;280:3757–3763. doi: 10.1074/jbc.M411325200. [DOI] [PubMed] [Google Scholar]
- 16.Petek M., Baebler S., Kuzman D., Rotter A., Podlesek Z., Gruden K., Ravnikar M., Urleb U. Revealing fosfomycin primary effect on Staphylococcus aureus transcriptome: Modulation of cell envelope biosynthesis and phosphoenolpyruvate induced starvation. BMC Microbiol. 2010;10:159. doi: 10.1186/1471-2180-10-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Falagas M.E., Vouloumanou E.K., Samonis G., Vardakasa K.Z. Fosfomycin. Clin. Microbiol. Rev. 2016;29:321–347. doi: 10.1128/CMR.00068-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raz R. Fosfomycin: An old-new antibiotic. Clin. Microbiol. Infect. 2012;18:4–7. doi: 10.1111/j.1469-0691.2011.03636.x. [DOI] [PubMed] [Google Scholar]
- 19.Liu H.Y., Lin H.C., Lin Y.C., Yu S.H., Wu W.H., Lee Y.J. Antimicrobial susceptibilities of urinary extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae to fosfomycin and nitrofurantoin in a teaching hospital in Taiwan. J. Microbiol. Immunol. Infect. 2011;44:364–368. doi: 10.1016/j.jmii.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 20.Bavaro D.F., Belati A., Diella L., Stufano M., Romanelli F., Scalone L., Stolfa S., Ronga L., Maurmo L., Dell’aera M., et al. Cefiderocol-based combination therapy for “difficult-to-treat” gram-negative severe infections: Real-life case series and future perspectives. Antibiotics. 2021;10:652. doi: 10.3390/antibiotics10060652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Butcu M., Akcay S.S., Inan A.S., Aksaray S., Engin D.O., Calisici G. In vitro susceptibility of enterococci strains isolated from urine samples to fosfomycin and other antibiotics. J. Infect. Chemother. 2011;17:575–578. doi: 10.1007/s10156-011-0212-7. [DOI] [PubMed] [Google Scholar]
- 22.Stracquadanio S., Musso N., Costantino A., Lazzaro L.M., Stefani S., Bongiorno D. Staphylococcus aureus internalization in osteoblast cells: Mechanisms, interactions and biochemical processes. what did we learn from experimental models? Pathogens. 2021;10:239. doi: 10.3390/pathogens10020239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reeves D.S. Fosfomycin trometamol. J. Antimicrob. Chemother. 1994;34:853–858. doi: 10.1093/jac/34.6.853. [DOI] [PubMed] [Google Scholar]
- 24.Arca P., Hardisson C., Suarez J.E. Purification of a glutathione S-transferase that mediates fosfomycin resistance in bacteria. Antimicrob. Agents Chemother. 1990;34:844–848. doi: 10.1128/AAC.34.5.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shrestha Nabin; Tomford JW Fosfomycin: A review. Infect. Dis. Clin. Pract. 2001;10:255–260. doi: 10.1097/00019048-200106000-00004. [DOI] [Google Scholar]
- 26.Kadner R.J., Winkler H.H. Isolation and characterization of mutations affecting the transport of hexose phosphates in Escherichia coli. J. Bacteriol. 1973;113:895–900. doi: 10.1128/jb.113.2.895-900.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kahan F.M., Kahan J.S., Cassidy P.J., Kropp H. The Mechanism of Action of Fosfomycin (Phosphonomycin) Ann. N. Y. Acad. Sci. 1974;235:364–386. doi: 10.1111/j.1749-6632.1974.tb43277.x. [DOI] [PubMed] [Google Scholar]
- 28.Tsuruoka T., Miyata A., Yamada Y. Two Kinds of Mutants Defective in Multiple Carbohydrate Utilization Isolated from in Vitro Fosfomycin-Resistant Strains of Escherichia Coli K-12. J. Antibiot. 1978;31:192–201. doi: 10.7164/antibiotics.31.192. [DOI] [PubMed] [Google Scholar]
- 29.Sakamoto Y., Furukawa S., Ogihara H., Yamasaki M. Fosmidomycin resistance in adenylate cyclase deficient (cya) mutants of Escherichia coli. Biosci. Biotechnol. Biochem. 2003;67:2030–2033. doi: 10.1271/bbb.67.2030. [DOI] [PubMed] [Google Scholar]
- 30.Venkateswaran P.S., Wu H.C. Isolation and characterization of a phosphonomycin-resistant mutant of Escherichia coli K-12. J. Bacteriol. 1972;110:935–944. doi: 10.1128/jb.110.3.935-944.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karageorgopoulos D.E., Wang R., Yu X.H., Falagas M.E. Fosfomycin: Evaluation of the published evidence on the emergence of antimicrobial resistance in gram-negative pathogens. J. Antimicrob. Chemother. 2012;67:255–268. doi: 10.1093/jac/dkr466. [DOI] [PubMed] [Google Scholar]
- 32.Rigsby R.E., Fillgrove K.L., Beihoffer L.A., Armstrong R.N. Fosfomycin resistance proteins: A nexus of glutathione transferases and epoxide hydrolases in a metalloenzyme superfamily. Methods Enzymol. 2005;401:367–379. doi: 10.1016/S0076-6879(05)01023-2. [DOI] [PubMed] [Google Scholar]
- 33.Garcia P., Arca P., Suarez J.E. Product of fosC, a gene from Pseudomonas syringae, mediates fosfomycin resistance by using ATP as cosubstrate. Antimicrob. Agents Chemother. 1995;39:1569–1573. doi: 10.1128/AAC.39.7.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhanel G.G., Walkty A.J., Karlowsky J.A. Fosfomycin: A First-Line Oral Therapy for Acute Uncomplicated Cystitis. Can. J. Infect. Dis. Med. Microbiol. 2016;2016:2082693. doi: 10.1155/2016/2082693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Falagas M.E., Athanasaki F., Voulgaris G.L., Triarides N.A., Vardakas K.Z. Resistance to fosfomycin: Mechanisms, Frequency and Clinical Consequences. Int. J. Antimicrob. Agents. 2019;53:22–28. doi: 10.1016/j.ijantimicag.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 36.Borsa F., Leroy A., Fillastre J.P., Godin M., Moulin B. Comparative pharmacokinetics of tromethamine fosfomycin and calcium fosfomycin in young and elderly adults. Antimicrob. Agents Chemother. 1988;32:938–941. doi: 10.1128/AAC.32.6.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bergan T. Degree of absorption, pharmacokinetics of fosfomycin trometamol and duration of urinary antibacterial activity. Infection. 1990;18:S65–S69. doi: 10.1007/BF01643430. [DOI] [PubMed] [Google Scholar]
- 38.Goto M., Sugiyama M., Nakajima S., Yamashina H. Fosfomycin kinetics after intravenous and oral administration to human volunteers. Antimicrob. Agents Chemother. 1981;20:393–397. doi: 10.1128/AAC.20.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roussos N., Karageorgopoulos D.E., Samonis G., Falagas M.E. Clinical significance of the pharmacokinetic and pharmacodynamic characteristics of fosfomycin for the treatment of patients with systemic infections. Int. J. Antimicrob. Agents. 2009;34:506–515. doi: 10.1016/j.ijantimicag.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 40.Joukhadar C., Klein N., Dittrich P., Zeitlinger M., Geppert A., Skhirtladze K., Frossard M., Heinz G., Müller M. Target site penetration of fosfomycin in critically ill patients. J. Antimicrob. Chemother. 2003;51:1247–1252. doi: 10.1093/jac/dkg187. [DOI] [PubMed] [Google Scholar]
- 41.Patel S.S., Balfour J.A., Bryson H.M. Fosfomycin Tromethamine. A review of its antibacterial activity, pharmacokinetic properties and therapeutic efficacy as a single-dose oral treatment for acute uncomplicated lower urinary tract infections. Drugs. 1997;53:637–656. doi: 10.2165/00003495-199753040-00007. [DOI] [PubMed] [Google Scholar]
- 42.Keating G.M. Fosfomycin trometamol: A review of its use as a single-dose oral treatment for patients with acute lower urinary tract infections and pregnant women with asymptomatic bacteriuria. Drugs. 2013;73:1951–1966. doi: 10.1007/s40265-013-0143-y. [DOI] [PubMed] [Google Scholar]
- 43.FDA Cder Monurol (Fosfomycin Tromethamine) Oral Suspension. [(accessed on 22 July 2022)]; Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/050717s005lbl.pdf.
- 44.Dijkmans A.C., Zacarías N.V.O., Burggraaf J., Mouton J.W., Wilms E.B., van Nieuwkoop C., Touw D.J., Stevens J., Kamerling I.M.C. Fosfomycin: Pharmacological, clinical and future perspectives. Antibiotics. 2017;6:24. doi: 10.3390/antibiotics6040024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fan L., Shang X., Zhu J., Ma B., Zhang Q. Pharmacodynamic and pharmacokinetic studies and prostatic tissue distribution of fosfomycin tromethamine in bacterial prostatitis or normal rats. Andrologia. 2018;50:e13021. doi: 10.1111/and.13021. [DOI] [PubMed] [Google Scholar]
- 46.EUCAST. MIC EUCAST. [(accessed on 9 May 2022)]. Available online: https://mic.eucast.org/search/?search%5Bmethod%5D=mic&search%5Bantibiotic%5D=100&search%5Bspecies%5D=-1&search%5Bdisk_content%5D=-1&search%5Blimit%5D=50.
- 47.Tosto F., Marino A., Moscatt V., Cosentino F., Campanella E., Micali C., Russotto Y., Caci G., Rullo E.V., Nunnari G., et al. Methicillin-sensitive Staphylococcus aureus prosthetic vascular graft infection after a Fontan procedure in an adult patient: A case report. World Acad. Sci. J. 2022;4:19. doi: 10.3892/wasj.2022.154. [DOI] [Google Scholar]
- 48.Castañeda-García A., Blázquez J., Rodríguez-Rojas A. Molecular mechanisms and clinical impact of acquired and intrinsic fosfomycin resistance. Antibiotics. 2013;2:217–236. doi: 10.3390/antibiotics2020217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mihailescu R., Tafin U.F., Corvec S., Oliva A., Betrisey B., Borens O., Trampuza A. High activity of fosfomycin and rifampin against methicillin-resistant staphylococcus aureus biofilm in vitro and in an experimental foreign-body infection model. Antimicrob. Agents Chemother. 2014;58:2547–2553. doi: 10.1128/AAC.02420-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chai D., Liu X., Wang R., Bai Y., Cai Y. Efficacy of Linezolid and Fosfomycin in Catheter-Related Biofilm Infection Caused by Methicillin-Resistant Staphylococcus aureus. Biomed Res. Int. 2016;2016:6413982. doi: 10.1155/2016/6413982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oliva A., Curtolo A., Volpicelli L., Cogliati Dezza F., De Angelis M., Cairoli S., Dell’utri D., Goffredo B.M., Raponi G., Venditti M. Synergistic meropenem/vaborbactam plus fosfomycin treatment of kpc producing k. Pneumoniae septic thrombosis unresponsive to ceftazidime/avibactam: From the bench to the bedside. Antibiotics. 2021;10:781. doi: 10.3390/antibiotics10070781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Flamm R.K., Rhomberg P.R., Lindley J.M., Sweeney K., Ellis-Grosse E.J., Shortridge D. Evaluation of the bactericidal activity of fosfomycin in combination with selected antimicrobial comparison agents tested against Gram-negative bacterial strains by using time-kill curves. Antimicrob. Agents Chemother. 2019;63:e02549-18. doi: 10.1128/AAC.02549-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Papp-Wallace K.M., Zeiser E.T., Becka S.A., Park S., Wilson B.M., Winkler M.L., D’Souza R., Singh I., Sutton G., Fouts D.E., et al. Ceftazidime-Avibactam in Combination with Fosfomycin: A Novel Therapeutic Strategy against Multidrug-Resistant Pseudomonas aeruginosa. J. Infect. Dis. 2020;221:666–676. doi: 10.1093/infdis/jiz149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cuba G.T., Rocha-Santos G., Cayô R., Streling A.P., Nodari C.S., Gales A.C., Pignatari A.C.C., Nicolau D.P., Kiffer C.R.V. In vitro synergy of ceftolozane/tazobactam in combination with fosfomycin or aztreonam against MDR Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2020;75:1874–1878. doi: 10.1093/jac/dkaa095. [DOI] [PubMed] [Google Scholar]
- 55.Samonis G., Maraki S., Karageorgopoulos D.E., Vouloumanou E.K., Falagas M.E. Synergy of fosfomycin with carbapenems, colistin, netilmicin, and tigecycline against multidrug-resistant Klebsiella pneumoniae, Escherichia coli, and Pseudomonas aeruginosa clinical isolates. Eur. J. Clin. Microbiol. Infect. Dis. 2012;31:695–701. doi: 10.1007/s10096-011-1360-5. [DOI] [PubMed] [Google Scholar]
- 56.Drusano G.L., Neely M.N., Yamada W.M., Duncanson B., Brown D., Maynard M., Vicchiarelli M., Louie A. The Combination of fosfomycin plus meropenem is synergistic for pseudomonas aeruginosa PAO1 in a hollow-fiber infection model. Antimicrob. Agents Chemother. 2018;62:e01682-18. doi: 10.1128/AAC.01682-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Leelasupasri S., Santimaleeworagun W., Jitwasinkul T. Antimicrobial Susceptibility among Colistin, Sulbactam, and Fosfomycin and a Synergism Study of Colistin in Combination with Sulbactam or Fosfomycin against Clinical Isolates of Carbapenem-Resistant Acinetobacter baumannii. J. Pathog. 2018;2018:3893492. doi: 10.1155/2018/3893492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao M., Bulman Z.P., Lenhard J.R., Satlin M.J., Kreiswirth B.N., Walsh T.J., Marrocco A., Bergen P.J., Nation R.L., Li J., et al. Pharmacodynamics of colistin and fosfomycin: A “treasure trove” combination combats KPC-producing Klebsiella pneumoniae. J. Antimicrob. Chemother. 2017;72:1985–1990. doi: 10.1093/jac/dkx070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee Y.C., Chen P.Y., Wang J.T., Chang S.C. Prevalence of fosfomycin resistance and gene mutations in clinical isolates of methicillin-resistant Staphylococcus aureus. Antimicrob. Resist. Infect. Control. 2020;9:135. doi: 10.1186/s13756-020-00790-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.M100Ed32|Performance Standards for Antimicrobial Susceptibility Testing, 32th Edition. [(accessed on 4 July 2022)]. Available online: https://clsi.org/standards/products/microbiology/documents/m100/
- 61.Van Mens S.P., ten Doesschate T., Kluytmans-van den Bergh M.F.Q., Mouton J.W., Rossen J.W.A., Verhulst C., Bonten M.J.M., Kluytmans J.A.J.W. Fosfomycin Etest for Enterobacteriaceae: Interobserver and interlaboratory agreement. Int. J. Antimicrob. Agents. 2018;52:678–681. doi: 10.1016/j.ijantimicag.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 62.Karlowsky J.A., Baxter M.R., Golden A.R., Adam H.J., Walkty A., Lagacé-Wiens P.R.S., Zhanel G.G. Use of Fosfomycin Etest To Determine In Vitro Susceptibility of Clinical Isolates of Enterobacterales Other than Escherichia coli, Nonfermenting Gram-Negative Bacilli, and Gram-Positive Cocci. J. Clin. Microbiol. 2021;59:e0163521. doi: 10.1128/JCM.01635-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Van Den Bijllaardt W., Schijffelen M.J., Bosboom R.W., Stuart J.C., DIederen B., Kampinga G., Le T.N., Overdevest I., Stals F., Voorn P., et al. Susceptibility of ESBL Escherichia coli and Klebsiella pneumoniae to fosfomycin in the Netherlands and comparison of several testing methods including Etest, MIC test strip, Vitek2, Phoenix and disc diffusion. J. Antimicrob. Chemother. 2018;73:2380–2387. doi: 10.1093/jac/dky214. [DOI] [PubMed] [Google Scholar]
- 64.Aprile A., Scalia G., Stefani S., Mezzatesta M.L. In vitro fosfomycin study on concordance of susceptibility testing methods against ESBL and carbapenem-resistant Enterobacteriaceae. J. Glob. Antimicrob. Resist. 2020;23:286–289. doi: 10.1016/j.jgar.2020.09.022. [DOI] [PubMed] [Google Scholar]
- 65.Campanile F., Wootton M., Davies L., Aprile A., Mirabile A., Pomponio S., Demetrio F., Bongiorno D., Walsh T.R., Stefani S., et al. Gold standard susceptibility testing of fosfomycin in Staphylococcus aureus and Enterobacterales using a new agar dilution panel®. J. Glob. Antimicrob. Resist. 2020;23:334–337. doi: 10.1016/j.jgar.2020.08.025. [DOI] [PubMed] [Google Scholar]
- 66.Parisio E.M., Camarlinghi G., Coppi M., Niccolai C., Antonelli A., Nardone M., Vettori C., Giani T., Mattei R., Rossolini G.M. Evaluation of the commercial AD fosfomycin test for susceptibility testing of multidrug-resistant Enterobacterales and Pseudomonas aeruginosa. Clin. Microbiol. Infect. 2021;27:788.e5. doi: 10.1016/j.cmi.2020.11.029. [DOI] [PubMed] [Google Scholar]
- 67.European Society of Clinical Microbiology and Infectious Diseases EUCAST: Clinical Breakpoints and Dosing of Antibiotics. [(accessed on 9 May 2022)]. Available online: https://www.eucast.org/clinical_breakpoints/
- 68.Martín-Gutiérrez G., Docobo-Pérez F., Rodríguez-Martínez J.M., Pascual A., Blázquez J., Rodriguez-Beltrán J. Detection of low-level fosfomycin-resistant variants by decreasing glucose-6-phosphate concentration in fosfomycin susceptibility determination. Antibiotics. 2020;9:802. doi: 10.3390/antibiotics9110802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cattoir V., Guérin F. How is fosfomycin resistance developed in Escherichia coli? Future Microbiol. 2018;13:1693–1696. doi: 10.2217/fmb-2018-0294. [DOI] [PubMed] [Google Scholar]
- 70.Los-Arcos I., Pigrau C., Rodríguez-Pardo D., Fernández-Hidalgo N., Andreu A., Larrosa N., Almirantea B. Long-Term Fosfomycin-Tromethamine Oral Therapy for Difficult-To-Treat Chronic Bacterial Prostatitis. Antimicrob. Agents Chemother. 2016;60:1854–1858. doi: 10.1128/AAC.02611-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Karaiskos I., Galani L., Sakka V., Gkoufa A., Sopilidis O., Chalikopoulos D., Alivizatos G., Giamarellou E. Oral fosfomycin for the treatment of chronic bacterial prostatitis. J. Antimicrob. Chemother. 2019;74:1430–1437. doi: 10.1093/jac/dkz015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Almeida F., Santos Silva A., Silva Pinto A., Sarmento A. Chronic prostatitis caused by extended-spectrum β-lactamase-producing Escherichia coli managed using oral fosfomycin—A case report. IDCases. 2019;15:e00493. doi: 10.1016/j.idcr.2019.e00493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Grayson M.L., Macesic N., Trevillyan J., Ellis A.G., Zeglinski P.T., Hewitt N.H., Gardiner B.J., Frauman A.G. Fosfomycin for Treatment of Prostatitis: New Tricks for Old Dogs. Clin. Infect. Dis. 2015;61:1141–1143. doi: 10.1093/cid/civ436. [DOI] [PubMed] [Google Scholar]
- 74.Cunha B.A., Gran A., Raza M. Persistent extended-spectrum β-lactamase-positive Escherichia coli chronic prostatitis successfully treated with a combination of fosfomycin and doxycycline. Int. J. Antimicrob. Agents. 2015;45:427–429. doi: 10.1016/j.ijantimicag.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 75.Denes E. Prolonged course of Fosfomycin-Trometamol for chronic prostatitis: An unknown good option. Scand. J. Urol. 2021;55:344–345. doi: 10.1080/21681805.2021.1933170. [DOI] [PubMed] [Google Scholar]
- 76.Gian J., Cunha B.A. Raoultella planticola chronic bacterial prostatitis with prostatic calcifications: Successful treatment with prolonged fosfomycin therapy. Int. J. Antimicrob. Agents. 2016;47:414. doi: 10.1016/j.ijantimicag.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 77.Marino A., Stracquadanio S., Ceccarelli M., Zagami A., Nunnari G., Cacopardo B. Oral fosfomycin formulation for acute bacterial prostatitis; a new role for an old molecule: A case report and brief literature review. World Acad. Sci. J. 2022;4:26. doi: 10.3892/wasj.2022.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shrestha N.K., Amuh D., Goldman M.P., Riebel W.J., Walton Tomford J. Treatment of a complicated vancomycinresistant enterococcal urinary tract infection with fosfomycin. Infect. Dis. Clin. Pract. 2000;9:368–371. doi: 10.1097/00019048-200009090-00004. [DOI] [Google Scholar]
- 79.Gill B.C., Shoskes D.A. Bacterial prostatitis. Curr. Opin. Infect. Dis. 2016;29:86–91. doi: 10.1097/QCO.0000000000000222. [DOI] [PubMed] [Google Scholar]
- 80.Clinical and Laboratory Standards Institute Clinical & Laboratory Standards Institute: CLSI Guidelines. [(accessed on 4 July 2022)]. Available online: https://clsi.org/
- 81.Magri V., Boltri M., Cai T., Colombo R., Cuzzocrea S., De Visschere P., Giuberti R., Granatieri C.M., Latino M.A., Larganà G., et al. Multidisciplinary approach to prostatitis. Arch. Ital. Urol. Androl. 2018;90:227–248. doi: 10.4081/aiua.2018.4.227. [DOI] [PubMed] [Google Scholar]
- 82.Demonchy E., Courjon J., Ughetto E., Durand M., Risso K., Garraffo R., Roger P.M. Cefoxitin-based antibiotic therapy for extended-spectrum β-lactamase-producing Enterobacteriaceae prostatitis: A prospective pilot study. Int. J. Antimicrob. Agents. 2018;51:836–841. doi: 10.1016/j.ijantimicag.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 83.Marino A., Caltabiano E., Zagami A., Onorante A., Zappalà C., Locatelli M.E., Pampaloni A., Scuderi D., Bruno R., Cacopardo B. Rapid emergence of cryptococcal fungemia, Mycobacterium chelonae vertebral osteomyelitis and gastro intestinal stromal tumor in a young HIV late presenter: A case report. BMC Infect. Dis. 2018;18:693. doi: 10.1186/s12879-018-3573-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Celesia B.M., Marino A., Del Vecchio R.F., Bruno R., Palermo F., Gussio M., Nunnari G., Cacopardo B. Is it safe and cost saving to defer the CD4+ cell count monitoring in stable patients on ART with more than 350 or 500 cells/µL? Mediterr. J. Hematol. Infect. Dis. 2019;11:e2019063. doi: 10.4084/mjhid.2019.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Celesia B.M., Marino A., Borracino S., Arcadipane A.F., Pantò G., Gussio M., Coniglio S., Pennisi A., Cacopardo B., Panarello G. Successful extracorporeal membrane oxygenation treatment in an acquired immune deficiency syndrome (AIDS) patient with acute respiratory distress syndrome (ARDS) complicating pneumocystis jirovecii pneumonia: A challenging case. Am. J. Case Rep. 2020;21:e919570-1–e919570-5. doi: 10.12659/AJCR.919570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Marino A., Cosentino F., Ceccarelli M., Moscatt V., Pampaloni A., Scuderi D., D’andrea F., Venanzi Rullo E., Nunnari G., Benanti F., et al. Entecavir resistance in a patient with treatment-naïve hbv: A case report. Mol. Clin. Oncol. 2021;14:113. doi: 10.3892/mco.2021.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.