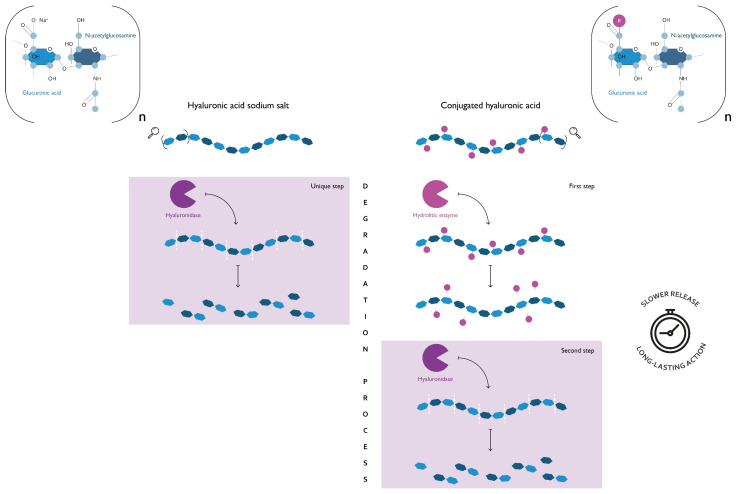
Figure 1.
A comparison of the degradation process of hyaluronan and conjugated hyaluronic acid (HA). Both polymers are composed of a variable number of dimers (n dimers) of glucuronic acid and N-acetylglucosamine, linked via alternating β-1,4 and β-1,3 glycosidic bonds. Hyaluronan is the sodium salt of hyaluronic acid (HA) and represents the form that naturally occurs in the human body. The conjugated HA is obtained through the introduction of chemical modifications of the HA backbone. As can be seen from this schematic representation, the presence of a covalently bonded functional group (R) has an impact on the polymer degradation process. If on one hand, the β-1,4 glycosidic bonds of hyaluronan are easily accessible for hyaluronidase, on the other, those of conjugated HA to be such require a preliminary step where an additional hydrolytic enzyme removes the functional group. This difference implies that the degradation products of conjugated HA are released over an extended period, thus ensuring a long-lasting action of the products that contain it. Vaginal moisturizers containing conjugated HA and, therefore, having a prolonged residence time on the mucosa, have been described by Cascone & Lamberti (2019) [85].