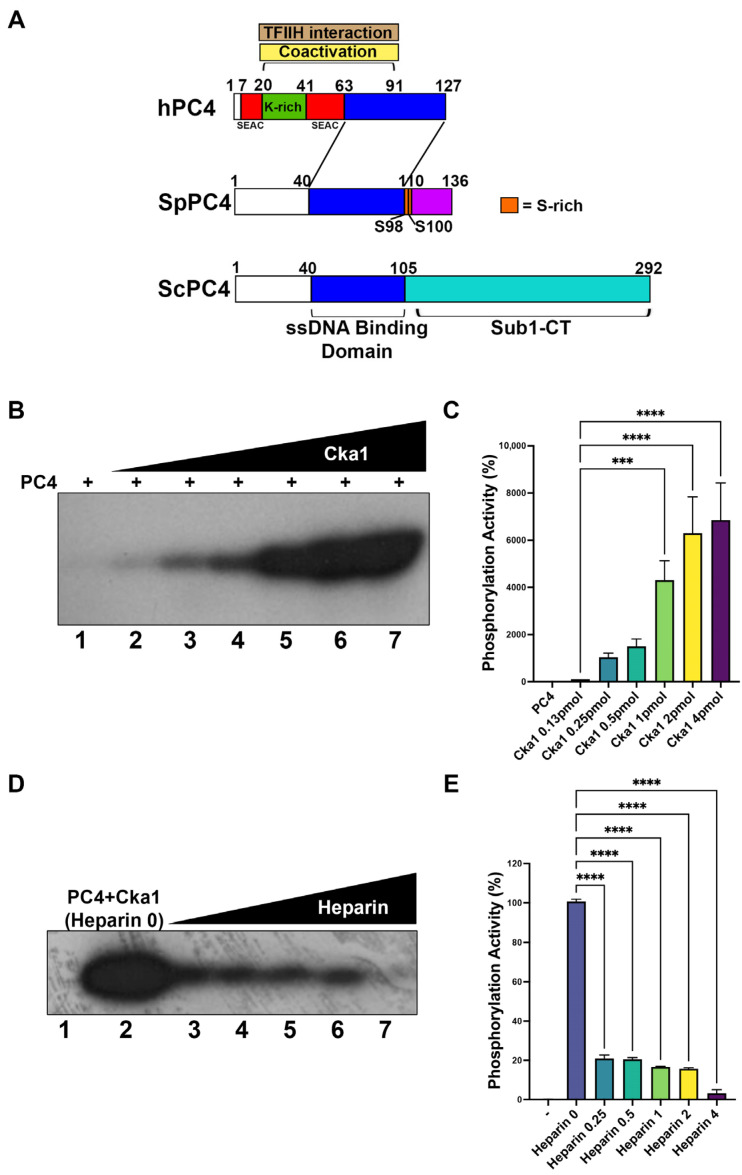
Figure 2.
Fission yeast Cka1 phosphorylates fission yeast PC4. (A) Schematic representation of human PC4 (hPC4), fission yeast PC4 (SpPC4) and yeast PC4 (ScPC4; Sub1) that shows the extension of the known structural domains. Human PC4 possesses two SEAC domains at the N-terminal portion, which are targets for CK2 phosphorylation. It also possesses a K-rich domain at the N-terminus and a TFIIH domain which overlaps the coactivation domain. All three possess a single-stranded DNA binding domain and at the same location the dimerization domain. Yeast Sub1 possesses an extra C-terminal domain of unknown function (purple region). The location of the mutated Ser residues of SpPC4 are shown (S98 and S100). (B) SpPC4 phosphorylation by Cka1. PC4 (500 ng) was phosphorylated with increasing amounts of Cka1 (lanes 2–7; 0.1, 0.25, 0.5, 1.0, 2.0 and 4.0 pmol of fission yeast Cka1). Negative lane 1 only contains PC4; + symbols indicate the addition of PC4 to the assays. (C) Signals of each lane from (B) were quantified and plotted using the Image J program. (D) Heparin at 0.25, 0.5, 1, 2 and 4 μg/mL (lanes 3–7) was used to inhibit the activity of Cka1. (E) Lanes from (D) were quantified and plotted using the Image J software; *** indicates p < 0.001, **** indicates p < 0.0001.